Long covid and the brain: Difference between revisions
| Line 39: | Line 39: | ||
==Pathophysiology== | ==Pathophysiology== | ||
The pathophysiology of COVID-19 as it affects the brain is complex and involves multiple potential mechanisms that can lead to neurological symptoms and complications. This field is still actively in research to investigating the exact pathways and mechanisms.[[Image:Brainm.png|thumb| | The pathophysiology of COVID-19 as it affects the brain is complex and involves multiple potential mechanisms that can lead to neurological symptoms and complications. This field is still actively in research to investigating the exact pathways and mechanisms.[[Image:Brainm.png|thumb|380px| Fig.3 ]] | ||
===Direct Invasion=== | ===Direct Invasion=== | ||
Coronavirus can directly invade the central neuron system(CNS) through direct hematogenous and neural propagation. Through this pathway, CoVs enters nasal pathway and can path epithelial barrier, especially blood-brain barrier(BBB), reaching the blood and lymph circulation and then propagated towards CN<ref>Desforges, M., Le Coupanec, A., Stodola, J.K., Meessen-Pinard, M. and Talbot, P.J. (2014). Human coronaviruses: Viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Research, 194, pp.145–158. doi:https://doi.org/10.1016/j.virusres.2014.09.011.</ref>. This is unusual because BBB is a highly selective and semipermeable border that separates the circulating blood from the brain and extracellular fluid in the central nervous system (CNS). The primary function of the BBB is to protect the brain from harmful substances in the blood and maintain a stable environment for the brain. It is composed of highly specialized endothelial cells that interact with pericytes, astrocytes, microglia, and neurons in the neurovascular unit and regulate the permeability of the BBB and maintain the integrity of the CNS. The potential pathway to enter CNS across the BBB was predicted in five ways: i) paracellular transport through leaky BBB due to disrupted tight junctions (TJs), ii) transcellular transport by direct infection of cerebrovascular endothelial cells, iii) transport via extracellular vesicles, a form of ‘Trojan horse’ trafficking, iv)transport via receptor-mediated endocytosis, v) transport via infected peripheral immune cells, another form of ‘Trojan horse’ trafficking<ref>Alam, S.B., Willows, S., Kulka, M. and Sandhu, J.K. (2020). Sever acute respiratory syndrome coronavirus‐2 may be an underappreciated pathogen of the central nervous system. European Journal of Neurology. doi:https://doi.org/10.1111/ene.14442.</ref>. | Coronavirus can directly invade the central neuron system(CNS) through direct hematogenous and neural propagation. Through this pathway, CoVs enters nasal pathway and can path epithelial barrier, especially blood-brain barrier(BBB), reaching the blood and lymph circulation and then propagated towards CN<ref>Desforges, M., Le Coupanec, A., Stodola, J.K., Meessen-Pinard, M. and Talbot, P.J. (2014). Human coronaviruses: Viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Research, 194, pp.145–158. doi:https://doi.org/10.1016/j.virusres.2014.09.011.</ref>. This is unusual because BBB is a highly selective and semipermeable border that separates the circulating blood from the brain and extracellular fluid in the central nervous system (CNS). The primary function of the BBB is to protect the brain from harmful substances in the blood and maintain a stable environment for the brain. It is composed of highly specialized endothelial cells that interact with pericytes, astrocytes, microglia, and neurons in the neurovascular unit and regulate the permeability of the BBB and maintain the integrity of the CNS. The potential pathway to enter CNS across the BBB was predicted in five ways: i) paracellular transport through leaky BBB due to disrupted tight junctions (TJs), ii) transcellular transport by direct infection of cerebrovascular endothelial cells, iii) transport via extracellular vesicles, a form of ‘Trojan horse’ trafficking, iv)transport via receptor-mediated endocytosis, v) transport via infected peripheral immune cells, another form of ‘Trojan horse’ trafficking<ref>Alam, S.B., Willows, S., Kulka, M. and Sandhu, J.K. (2020). Sever acute respiratory syndrome coronavirus‐2 may be an underappreciated pathogen of the central nervous system. European Journal of Neurology. doi:https://doi.org/10.1111/ene.14442.</ref>. | ||
Revision as of 00:22, 15 April 2024
About SARS-CoV-2 virus and Long COVID
By Hao Yang
SARS-CoV-2
SARS-CoV-2, or severe acute respiratory syndrome coronavirus 2, is the virus responsible for the global pandemic that began in late 2019. It is the causative agent of the disease known as COVID-19 (Coronavirus Disease 2019). The virus first emerged in the city of Wuhan, Hubei province, China, and has since spread worldwide, leading to significant health, economic, and social impacts.The two highly pathogenic zoonotic origin virus from this family that appeared in 2002 and 2012 caused Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), leading to a wild range of public health crisis[1]. These viruses are zoonotic which means they can be transmitted between animals and humans. The novel coronavirus emerged in 2019 designated as SARS-CoV-2 is far more contagious compare to other coronavirus before.
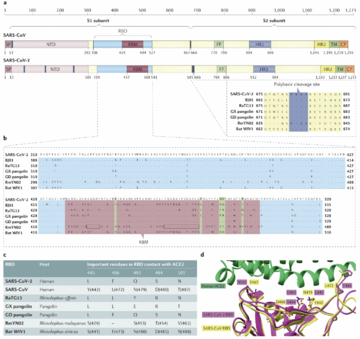
SARS-CoV-2 belongs to the Coronaviridae family, a group of viruses that can cause illnesses ranging from the common cold to more severe diseases. They are enveloped, positive-strand RNA viruses. The virus is characterized by its crown-like appearance under an electron microscope due to the presence of spike proteins on its surface. These spike proteins are crucial for the virus as they allow it to attach to and enter human cells through the ACE2 receptor, primarily found in epithelial cells for lung tissue but also in other tissues such as the heart, kidneys, and intestines. The genome sequencing research found SARS-CoV-2 shares 79% of its genome with SARS-CoV and 50% with MERS-CoV. The most protein encoding for replicase(ORF1a/ORF1b), envelope, membrane, and nucleocapsid are very similar, except the spike protein [2]. The phylogenetic analysis for SARS-CoV-2 genome shows it clustered with SARS-CoV and SARS-related coronaviruses (SARSr-CoVs) found in bats. Therefore, SARS-CoV-2 was placing at subgenus Sarbecovirus of the genus Betacoronavirus. This founding also lead to the discussion that SARS-CoV-2 may have originated in bats and possibly involved another intermediary transitions among other animals before it infected human.
SARS-CoV-2's genome is longer than both SARS-CoV and known bat SARSr-CoVs. After genomic comparison, researches found there is an insertion of four amino acid residues(PRRA) at the junction of subunits S1 and S2 of the spike protein. This insertion mutation might explained the increasing infectiousness of COVID-19. In addition, a structural study suggested that the furin-cleavage site can reduce the stability of SARS-CoV-2 S protein and facilitate the conformational adaption that is required for the binding of the RBD to its receptor[3].
Long COVID
Long COVID or Post COVID conditions, also known as post-acute sequelae of SARS-CoV-2 infection(PASC),refers to a range of symptoms that continue for weeks, months, or even years after the patient initially recovering from the coronavirus disease(COVID-19). The condition can affect anyone who was infected by COVID-19, regardless of the severity of their initial infection. The symptoms of long term COVID can various between each individual and be very complicated because it sometimes impact multiple organism systems. The complexity of the symptoms often leads to post-COVID conditions being diagnosed as other diseases.
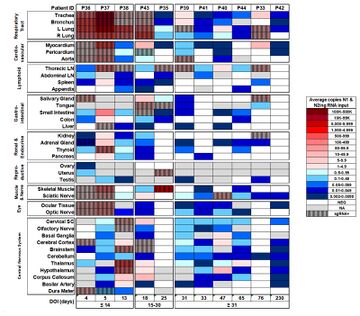
Although COVID is primarily target to respiration systems, there is growing evidence that COVID-19 can have long-term effects on the brain, including potential links to increased risks of neurological diseases like Parkinson's and Alzheimer's disease in the future. Corona virus was unusual because it had been found the virus across blood-brain barrier. However, the detection of coronaviral RNA in human brain samples clearly demonstrates that these respiratory pathogens are naturally neuroinvasive in humans and suggests that they establish a persistent infection in human CNS. The dissecting samples in patients also shows the virus is preserved in brain tissue over months and it is still active[4].
Neurological Symptoms
Diagnosis and Evaluation
Diagnose Long COVID is an exclusion process, indicating that it is only diagnosed after doctor excluding all the possible disease or causes that lead the symptoms. It is a tricky process that for sometimes doctors will reveal some hidden diseases that even the patient doesn't know before. In order to make precise diagnose, it always need to go through a comprehensive diagnose process including physical examination, laboratory test and imaging such as blood test, chest X-rays, cardiac testing, pulmonary function test, multidisciplinary approaches such as psychiatric diagnose if necessary. Due to the various symptoms patient experienced, there is no standard treatment for long COVID.
Common Symptoms
Brain Fog: Brain fog a range of neuron-cognitive symptoms that including forgetfulness, problem of focusing, concentrating, and paying attentions. These symptoms can last for weeks and months after the patient get initial infections[5].
Sleep Disturbance: Approximately 40% of people with long COVID reported major changes of their sleep after COVID-19. People have experienced COVID-19-associated sleep disorders including insomnia and restless leg syndrome[6][7].
Anxiety,depression, and stress:People who have had COVID-19 may experience mood disorders including constantly feeling anxiety, depression, and stress.
Post-exertional malaise:Post-exertional malaise is one of the most common symptoms patient experience after recovered from COVID-19. People described they feeling very tired after only a little bit cognitive, physical, social, and emotional activity. Persistent fatigue is also accompanied by a decrease in physical stamina, leading to increased sleepiness.
Sensitivity and Pain:People with Long COVID may experience new pain or loss of sensitivity to touch. The gain of pain also known as post-COVID pain They also may experience tingling or burning sensations. These changes tend to be worse in the limbs, particularly in the hands, and/or feet. This is because COVID-19 is thought to affect the nerves that send signals like touch, pressure, heat/cold, and pain to the brain.[8]
Headache:Headache is one of the most common neuropsychiatric symptoms patient reported. There are about 44% patients report headache after get infected. [9]
Dizziness and Fainting:After recover from COVID-19, the part of the nervous system that regulates involuntary functions such as blood transportation and blood pressure might not completely recover. This is also known as dysautonomia and can lead to increase heart rate and fainting when standing up from a lying down or sitting position. According NIH, this type of dizziness and fainting also called postural orthostatic tachycardia syndrome (POTS).
Movement issue:Motor disorders caused by infections of the brain might also happened since it impact the neutron signaling and body coordination. Some people with long COVID have trouble with coordination (ataxia), loss of movement (bradykinesia), tremor, or sudden muscle twitching or jerking (myoclonus).
Other severe symptoms:More severe neurological syndromes also happened on patients including encephalopathies, para- and post-infectious CNS syndromes including encephalitis, ADEM with haemorrhage and necrotic change, transverse myelitis, ischaemic stroke and GBS[10]. Some other patients may also experience various symptoms including partial epilepsy, and mild cognitive impairment[11].
Pathophysiology
The pathophysiology of COVID-19 as it affects the brain is complex and involves multiple potential mechanisms that can lead to neurological symptoms and complications. This field is still actively in research to investigating the exact pathways and mechanisms.
Direct Invasion
Coronavirus can directly invade the central neuron system(CNS) through direct hematogenous and neural propagation. Through this pathway, CoVs enters nasal pathway and can path epithelial barrier, especially blood-brain barrier(BBB), reaching the blood and lymph circulation and then propagated towards CN[12]. This is unusual because BBB is a highly selective and semipermeable border that separates the circulating blood from the brain and extracellular fluid in the central nervous system (CNS). The primary function of the BBB is to protect the brain from harmful substances in the blood and maintain a stable environment for the brain. It is composed of highly specialized endothelial cells that interact with pericytes, astrocytes, microglia, and neurons in the neurovascular unit and regulate the permeability of the BBB and maintain the integrity of the CNS. The potential pathway to enter CNS across the BBB was predicted in five ways: i) paracellular transport through leaky BBB due to disrupted tight junctions (TJs), ii) transcellular transport by direct infection of cerebrovascular endothelial cells, iii) transport via extracellular vesicles, a form of ‘Trojan horse’ trafficking, iv)transport via receptor-mediated endocytosis, v) transport via infected peripheral immune cells, another form of ‘Trojan horse’ trafficking[13].
The other route of invasion by Coronavirus into CNS is through neural dissemination due to the polarization of neurons. This transport can be retrograde or antegrade and is facilitated by dynein and kinesin. It may leads to hyposmia in patients due to "conductive" loss or "neuron" loss. Depending on the recovery period, the patient can determines wether it is "conductive" loss or "neuron" loss. Other researches demonstrated that both angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 are primarily found in the non-neuronal cells of the olfactory epithelium and olfactory bulb in mice and humans. Additionally, neuropilin-1 (NRP1) has been identified in the olfactory epithelium. Thus, it's suggested that ACE2 and NRP1 could play roles in how SARS-CoV-2 is transmitted from the olfactory nerves to the central nervous system (CNS). Moreover, SARS-CoV-2 can infect both mature and immature olfactory neurons, as well as sustentacular supporting cells in hamsters, potentially explaining the distinct loss of smell observed in COVID-19[14]. Despite the olfactory bulb's role in early viral control, numerous studies have established the olfactory route as a significant pathway for the virus to enter the CNS[15].
ACE2 receptor
Like SARS-CoV, SARS-CoV-2 have the ability to bind to the protein called "Angiotensin-converting enzyme” (ACE2), or angiotensin-converting enzyme of the second type (ACE2). ACE2, a zinc-containing metalloenzyme and carboxypeptidase, is found on the surface of endothelial and various other cells as an ectoenzyme. The insertion mutation on spike protein for SARS-CoV-2 is more specifically in recognizing ACE2 and has a stronger binding affinity with ACE2 due to the presence of a receptor-binding domain[16]. ACE2 is widely expressing in variety of cells in lungs, kidneys, cardiovascular systems, guts and CNS. It serves important functions to regulates blood pressure, fluid and electrolyte balance, and systemic vascular resistance. The wild spread of ACE2 receptor implying SARS-CoV-2 can impact multiple organs and systems, triggering complex symptoms. In the brain, ACE2 is expressed in the neurons, astroglia cells, microglia cells, and endothelial cells. ACE2 is a negative regulator of the renin–angiotensin system, all components of which are present in the brain. Depletion of ACE2 increases the expression of angiotensin II, leading to vasoconstriction, sodium and water retention, elevated blood pressure, proinflammatory, and procoagulation effects. Overall, ACE2 is the most well-known and popular pathway that SARS-CoV-2 invade CNS. Preventing SARS-CoV-2 binding with ACE2 may be a potential therapeutic strategies to preventing COVID.
COVID and the Nervous System
Given COVID's ability to invade the nervous system and its characteristics of persistent survival and transcription in parts of the brain, an increasing number of researchers are beginning to focus on the impact of COVID-19 on long-term neurodegenerative diseases.
Alzheimer's disease
Alzheimer's disease(AD) is a progressive neurodegenerative disorder that primarily affects the elderly, though early-onset forms of the disease can begin much earlier. It is the most common cause of dementia among older adults and is characterized by a decline in cognitive function and memory. The symptoms of Alzheimer's disease develop gradually and worsen over time, eventually interfering with daily tasks and independent living. The brain of AD patients is characterized by amyloid plaque deposition and the presence of neurofibrillary tangles, which induce neuronal damage and synapse loss as well as oligodendroglia degeneration and myelin impairment[17].
The key foctor correlates SARS-CoV-2 and the Alzheimer's disease is the ACE2 expression, which tightly impact the viral invasion of CNS system. ACE2 is expressed at front lobe and hippocampus which represent cerebral regions involved in the pathogenesis of AD[18]. Post-mortem studies showed that ACE2 expression is increased in the brain of AD patients in comparison to controls. Moreover, genome-wide association studies (GWAS) showed that the expression of ACE2 gene is elevated in the brain tissue of AD patients with increased levels in severe forms. The enhanced ACE-2 expression could represent a risk factor for COVID-19 transmission among AD patients.
On the other side, recent studies suggests elder COVID patients is more susceptible to neuroninvasion during COVID infection because the integrity of BBB is lose which leaves larger gap for virus to enter. SARS CoV-2 infects the olfactory neurons and, through the neuro-epithelium of the olfactory mucosa, reaches the olfactory bulb in the hypothalamus[19]. The presence of SARS CoV-2 in the olfactory bulb leads to the activation of non-neuronal cells, such as mast cells, microglia, astrocytes, as well as to the tissue release of pro-inflammatory cytokines. One of the unique characteristic of SARS-CoV-2 replication is that they will use host endoplasmic reticulum to build its own envelope. The consequence is that the cells, in particular the innate immune cells, lose precursors for the synthesis of the autacoid local injury antagonist amides (ALIamides), which have a pivotal role for controlling the excessive reactivity[20]. The loss of functional protein leads to uncontrollable neuroninflammation, especially in elder patients, which have a weaker and less efficient immune system. Neuroinflammation is associated with intense oxidative stress, could induce neurodegeneration, potentially favoring the development of neurodegenerative diseases, such as AD. Therefore, COVID-19 patients with advanced age and comorbidities with an inflammatory basis, such as diabetes, atherosclerosis and sub-clinical dementia, could be at increased risk of developing AD. Beside that, A growing body of evidence suggested a role for neuroinflammation. Systemic inflammation induces the activation of microglia and astrocytes, which in turn secrete pro-inflammatory cytokines, including IL-1β, IL-6, IL-12, TNF-α. Such biomarkers could be involved in the synaptic dysfunction, inducing neurodegeneration, which could potentially lead to AD[21]. In conclusion, neuron impairment by SARS-CoV-2 invasion result the increasing of risks of AD now known have four potential pathway ways: i)Aß deposition
All these clinical case studies and analysis is short term, the long-term complications of COVID-19 would be expected in the next 10–15 years. Nowadays, it is not possible to assess them because the pandemic started last year. However, in the future, it will be pivotal to evaluate the risk of long-term COVID-19 neurological sequelae, especially in the elderly and patients who developed severe forms.
Parkinson's disease
Parkinson's disease is a progressive neurological disorder that primarily affects movement. It develops gradually, sometimes starting with a barely noticeable tremor in just one hand. While tremors are a common sign of Parkinson's disease, the disorder also commonly causes stiffness or slowing of movement. Over time, Parkinson's disease can affect not only motor skills but also speech and cognitive functions.
Treatment and Management
References
- ↑ Peeri, N.C., Shrestha, N., Rahman, M.S., Zaki, R., Tan, Z., Bibi, S., Baghbanzadeh, M., Aghamohammadi, N., Zhang, W. and Haque, U. (2020). The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? International Journal of Epidemiology, [online] 49(3). doi:https://doi.org/10.1093/ije/dyaa033.
- ↑ Hu, B., Guo, H., Zhou, P. and Shi, Z.-L. (2020). Characteristics of SARS-CoV-2 and COVID-19. Nature Reviews Microbiology, [online] 19(19), pp.1–14. doi:https://doi.org/10.1038/s41579-020-00459-7.
- ↑ Wrobel, A.G., Benton, D.J., Xu, P., Roustan, C., Martin, S.R., Rosenthal, P.B., Skehel, J.J. and Gamblin, S.J. (2020). SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nature Structural & Molecular Biology, [online] pp.1–5. doi:https://doi.org/10.1038/s41594-020-0468-7.
- ↑ Chertow, D., Stein, S., Ramelli, S., Grazioli, A., Chung, J.-Y., Singh, M., Yinda, C.K., Winkler, C., Dickey, J., Ylaya, K., Ko, S.H., Platt, A., Burbelo, P., Quezado, M., Pittaluga, S., Purcell, M., Munster, V., Belinky, F., Ramos-Benitez, M. and Boritz, E. (2021). SARS-CoV-2 infection and persistence throughout the human body and brai. [online] Europe PMC. Available at: https://europepmc.org/article/ppr/ppr434451.
- ↑ covid19.nih.gov. (n.d.). Shining a Light on Long COVID Brain Fog | NIH COVID-19 Research. [online] Available at: https://covid19.nih.gov/news-and-stories/shining-light-long-covid-brain-fog#:~:text=%E2%80%9CBrain%20fog%E2%80%9D%20is%20a%20range.
- ↑ Tony, A.A., Tony, E.AE., Ali, S.B., Ezzeldin, A.M. and Mahmoud, A.A. (2020). COVID-19-associated sleep disorders: A case report. Neurobiology of Sleep and Circadian Rhythms, 9, p.100057. doi:https://doi.org/10.1016/j.nbscr.2020.100057.
- ↑ www.ninds.nih.gov. (n.d.). COVID-19 and the Nervous System | National Institute of Neurological Disorders and Stroke. [online] Available at: https://www.ninds.nih.gov/current-research/coronavirus-and-ninds/covid-19-and-nervous-system#:~:text=Since%20COVID%2D19%20can%20affect.
- ↑ Fernández-de-las-Peñas, C., Herrero-Montes, M., Cancela-Cilleruelo, I., Rodríguez-Jiménez, J., Parás-Bravo, P., Varol, U., del-Valle-Loarte, P., Flox-Benítez, G., Arendt-Nielsen, L. and Valera-Calero, J.A. (2022). Understanding Sensitization, Cognitive and Neuropathic Associated Mechanisms behind Post-COVID Pain: A Network Analysis. Diagnostics, 12(7), p.1538. doi:https://doi.org/10.3390/diagnostics12071538.
- ↑ Lopez-Leon, S., Wegman-Ostrosky, T., Perelman, C., Sepulveda, R., Rebolledo, P.A., Cuapio, A. and Villapol, S. (2021). More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Scientific Reports, [online] 11(1), p.16144. doi:https://doi.org/10.1038/s41598-021-95565-8.
- ↑ Paterson, R.W., Brown, R.L., Benjamin, L., Nortley, R., Wiethoff, S., Bharucha, T., Jayaseelan, D.L., Kumar, G., Raftopoulos, R.E., Zambreanu, L., Vivekanandam, V., Khoo, A., Geraldes, R., Chinthapalli, K., Boyd, E., Tuzlali, H., Price, G., Christofi, G., Morrow, J. and McNamara, P. (2020). The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain, 143(10). doi:https://doi.org/10.1093/brain/awaa240.
- ↑ Helms, J., Kremer, S., Merdji, H., Clere-Jehl, R., Schenck, M., Kummerlen, C., Collange, O., Boulay, C., Fafi-Kremer, S., Ohana, M., Anheim, M. and Meziani, F. (2020). Neurologic Features in Severe SARS-CoV-2 Infection. New England Journal of Medicine, 382(23). doi:https://doi.org/10.1056/nejmc2008597.
- ↑ Desforges, M., Le Coupanec, A., Stodola, J.K., Meessen-Pinard, M. and Talbot, P.J. (2014). Human coronaviruses: Viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Research, 194, pp.145–158. doi:https://doi.org/10.1016/j.virusres.2014.09.011.
- ↑ Alam, S.B., Willows, S., Kulka, M. and Sandhu, J.K. (2020). Sever acute respiratory syndrome coronavirus‐2 may be an underappreciated pathogen of the central nervous system. European Journal of Neurology. doi:https://doi.org/10.1111/ene.14442.
- ↑ Zhang, A.J., Lee, A.C.-Y., Chu, H., Chan, J.F.-W., Fan, Z., Li, C., Liu, F., Chen, Y., Yuan, S., Poon, V.K.-M., Chan, C.C.-S., Cai, J.-P., Wu, K.L.-K., Sridhar, S., Chan, Y.-S. and Yuen, K.-Y. (2020). Severe Acute Respiratory Syndrome Coronavirus 2 Infects and Damages the Mature and Immature Olfactory Sensory Neurons of Hamsters. Clinical Infectious Diseases, 73(2), pp.e503–e512. doi:https://doi.org/10.1093/cid/ciaa995.
- ↑ Durrant, D.M., Ghosh, S. and Klein, R.S. (2016). The Olfactory Bulb: An Immunosensory Effector Organ during Neurotropic Viral Infections. ACS Chemical Neuroscience, 7(4), pp.464–469. doi:https://doi.org/10.1021/acschemneuro.6b00043.
- ↑ Shang, J., Ye, G., Shi, K., Wan, Y., Luo, C., Aihara, H., Geng, Q., Auerbach, A. and Li, F. (2020). Structural basis of receptor recognition by SARS-CoV-2. Nature, 581(7807). doi:https://doi.org/10.1038/s41586-020-2179-y.
- ↑ Papuć, E. and Rejdak, K., 2018. The role of myelin damage in Alzheimer’s disease pathology. Archives of Medical Science, 16(2), pp.345-341.
- ↑ Dong, M., Zhang, J., Ma, X., Tan, J., Chen, L., Liu, S., Xin, Y. and Zhuang, L. (2020). ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomedicine & Pharmacotherapy, 131, p.110678. doi:https://doi.org/10.1016/j.biopha.2020.110678.
- ↑ Steardo, L., Steardo Jr, L., Zorec, R. and Verkhratsky, A., 2020. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID‐19. Acta Physiologica (Oxford, England), 229(3).
- ↑ Yan, B., Freiwald, T., Chauss, D., Wang, L., West, E., Bibby, J., Olson, M., Kordasti, S., Portilla, D., Laurence, A. and Lionakis, M.S., 2020. SARS-CoV2 drives JAK1/2-dependent local and systemic complement hyperactivation. Research Square.
- ↑ Mohammadi, S., Moosaie, F. and Aarabi, M.H. (2020). Understanding the Immunologic Characteristics of Neurologic Manifestations of SARS-CoV-2 and Potential Immunological Mechanisms. Molecular Neurobiology. doi:https://doi.org/10.1007/s12035-020-02094-y.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024