Magnetospirillum magnetotacticum
Classification
Bacteria (Kingdom); Proteobacteria (Phylum); Alphaproteobacteria (Class); Rhodospirillales (Order); Rhodospirillaceae (Class); Magnetospirillum (Family); Magnetospirillum (Genus) [7]
Species
Magnetospirillum magnetotacticum (Synonym: Aquaspirillum magnetotacticum)
Description and Significance
Magnetospirillum magnetotacticum is a helical, magnetotactic, microaerophilic spirillum. It is typically isolated from the oxic-anoxic transition zone at the sediment-water interface of freshwater environments [1], [6]. First identified by Robert Blakemore in the 1980’s, this microbe was initially called Aquaspirillum magnetotacticum. Later phylogenetic studies of the 16sRNA sequence resulted in the creation of a new and distinct genus, Magnetospirillum, under which A. magnetotacticum was renamed [2]. It is a member of the magnetotactic bacteria (MTB) class, whose mobility is dependent upon the magnetic characteristics of its environment[6] M. magnetotacticum remains one of the most important species of Magnetospirillum as it was the first to be discovered and is one of the few that is culturable [3]. To this day, it remains on of the best studied of the taxonomically and morphologically diverse MTB class [10]. This is a biologically, ecologically, and commercially relevant microbe that synthesizes high quality single magnetite crystals far superior to those produced industrially. Some of its many potential uses including: evolutionary for magnetotaxis; creation of models for biomineralization; geobiological tracers; mineralization of sediment, bioremediation; contrast enhances in MRI's; waste water treatments for removal of heavy metals and radionuclides; cell separation imaging; manufacturing of printing inks and magnetic tapes; and magnetic targeting of pharmaceuticals [4], [3]. Because many of the biosynthetic pathways employed by this microbe have yet to be discovered, it remains an active area of research. Plans to utilize the valuable magnetite from these organisms will require a better understanding of their various metabolic processes and nutritional requirements [3]. Current research is aimed at completing the genome sequence of M. magnetotacticum. This will allow for further exploration of phylogenetic relationships and could lead to the identification of the magnetite biosynthetic genes [3].
Genome Structure
Magnetospirillum Magnetotacticum has a circular genome, consisting of a total of 9,211,536 base pairs, with a G-C content of 66.43% . Protein coding genes comprise 99.08% of the genome, the rest coding for rRNA genes. There are no pseudogenes present in the genome. Though no extrachromosomal structures are found when analyzing undigested DNA, one study found possible evidence of circular plasmid of 40,000 base pairs that contains 16s rRNA, bra and por genes, made linear and thus detectable by enzyme digestion. An interesting feature of the chromosome is the clustering of genes that contribute this microbes magnetotaxicity. The genes bfr (coding for the iron the iron storage protien bacterioferritin) and magA (coding for the magnetosomal mambrane that surrounds magnetite crystals), are located maximally in the same 17% of the genome, though further studies expect to find that they are indeed much closer together. Also interesting is the overlapping of two bfr genes. Very few magnetotactic bacteria code for two bfr genes, and the few that do, have no overlap. The reason for the overlap is possibly to keep the two genes in proximity, or could play a role in the regulation of the amounts of bacterioferritin protein produced. Magnetospirillum Magnetotacticum was the first magnetotactic bacteria to be phylogenetically analyzed using 16s rRNA genes. This is because it was one of the first magnetotactic bacteria to be isolated in pure culture. Origional analysis placed the bacteria in the Aquaspirillum genus, however further studies have placed it in a new genus of Magnetospirillum. This study resulted in the following evolutionary distance tree.
Cell Structure, Metabolism and Life Cycle
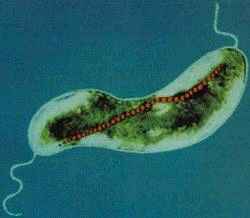
M. magnetotacticum is a gram negative, helical (clockwise), motile, spirilla that possesses two bipolar flagella. The organism ranges from 0.2-0.4 by 4.0-6.0µm and exhibits a wavelength of 1-2µm. M. magnetotacticum is a freshwater, denitrifying, chemoheterotroph[1], [10]. It can fix (N2) by reducing acetylene under microaerophilic conditions [10]. M.Magnetotacticum is a well adapted microbe capable of utilizing a broad range of tricarboxylic acid cycle intermediates and carbohydrates as both carbon and energy sources [1]. It is similarly able to select among co-oxidants: O2, NO3-, or FeIII as electron acceptors [10]. How iron is acquired so efficiently by the organism in its iron poor environment remains an area of debate [4]. Previous studies suggesting that M. magnetotacticum produces a hydroxamate siderophore for iron acquisition, have been largely irreproducible [11], [4]. What is know, is that the microbe does readily acquire iron and that iron concentrations make up approximately 2% of the cells total dry weight [11]. With the low levels of iron in the environment of M. magnetotacticum, it is inferred that highly specialized and efficient iron aquisition mechanisms are at work [4]. It is believed that iron, FeIII, reduction is coupled with iron acquisition, needed during intracellular magnetite (Fe3O4) crystal formation. The mechanism for magnetite biosynthesis is unknown [1], [3], [4]. Although, one proposed mechanism involves the reductive uptake of FeIII and subsequent reoxidiztion to a low density hydorous oxide, followed by dehydration to form the high density compound ferrihydrite [4]. The magnetite crystals synthesized by M. magnetotacticum are packaged into membrane enclosed compartments called magnetosomes. Magnetosomes have a cubooctahedral morphology and typically range in size from 40-30nm [1], [4]. They are arranged in chains along the long axis of the cell and act cooperatively as a geomagnetic navigational apparatus [10], [4]. The magnetosomes are responsible for establishing a magnetic dipole in the organism that allows for efficient detection and migration along the Earth's geomagnetic gradient [10]. The evolutionary advantages of magnetotaxis are not well understood. However, some speculate that magnetotaxis allows the microbe to traverse its environment in a more predictable and organized manner than Brownian movement allows [5]. It may also serve as a mechanism by which the bacteria can properly orient themselves in an optimal environment with reduced oxygen levels and high reduction potential. Other proposed uses of intracellular iron uptake in M. magnetotacticum include: redox cycling, functions in iron homeostasis, and energy conservation [4]. As a member of the bacterial kingdom, M. magnetotacticum divides via binary fission, in which one parent cell gives rise to two identical daughter cells. These bacteria generally form long chains of helical cells. In older cultures, coccoid bodies are abundant [1].
Ecology and Pathogenesis
Magnetospirillum Magnetotacticum was first found by R.P. Blakemore in 1975, isolated from the microarobic zone of a pond. These bacteria, as well as many other magnetotactic bacteria, live in the sediments of fresh water or wet soils. They predominate at the water-sediment interface, an oxygen concentration transition zone, which is consistent with there microaerophillic (sometimes anaerobic) lifestyle. Optimal oxygen conditions for Magnetospirillum magnetotacticum is 3-5% saturation. The environment also has an abundance of dissolved organic acids, utilized by the organism for energy. The main contribution of M. magnetotacticum to the environment is its production of magnetosomes, and the magnetofossils that are preserved after the death of the organism. These particles of magnetite significantly contribute to the magnetization of soils and sediments. Magnetospirillum magnetotacticum also fixes nitrogen, contributing to the availability of nitrogen in wet soils and sediments. As far as current research goes, Magnetospirillum magnetotacticum has no important symbiotic relationships. This organism is of biogeochemical significance because of its role in iron cycling. It is especially of interest because, understanding the process by which magnetite is formed, along with the overall metabolism can illustrate how the biogeochemical cycles of nitrogen, carbon and iron can be influenced by one organism. Magnetospirillum magnetotacticum is of geological significance because it leaves a detectable fossil remain, and can be used as a geobiological tracer.
References
- Maratea, D., and Blakemore, R.P., 1981. Aquaspirillum magnetotacticum sp. nov., a Magnetic Spirillum. International Journal of Systematic Bacteriology. Vol 31.4:452-455.
- Brenner,D. J., Bergey, D. H., Garrity, G. M., Krieg, N. R., Bergey's manual of systematic bacteriology Ed.2.Springer.2005. p:30-31.
- "Magnetospirillum magnetotacticum" http://genome.jpi-psf.org/draft-microbes/magma/magma.home.html
- Schülwer, D., 1999. Formation of Magnetosomes in Magnetotactic Bacteria. Journal of Molecular Microbiology and Biotechnology. 1(1):79-86
- Blakemore, R.P., and Frankel. 1981. Magnetite and magnetotaxis in microorganisms. Bioelectromagnetics. Vol 10.3:223-237
- "Magnetotactic bacteria" http://en.wikipedia.org/wiki/Magnetotactic_bacteria
- "Magnetosprillum magnetotacticum MS-1": http://www.uniprot.org/taxonomy/188
- "Magnetosprillum magnetotacticum MS-1": http://genamics.com/cgi-bin/genamics/genomes/genomesearch.cgi?field=ID&query=955
- Eden, P., Schmidt, T., Blakemore, R., & Pace, N. 1991. Phylogenetic Analysis of Aquaspirillum magnetotacticum Using Polymerase Chain Reaction-Amplified 16S rRNA-Specific DNA. International Journal of Systematic Bacteriology. Vol 4.2:324-325.
- Guerin, W., & Blakemore, R. 1992. Redox Cycling of Iron SUpports Growth and Magnetic Synthesis of Aquaspirillum magnetotacticum. Applied & Envirnmental Microbiology. Vol 58.4:1102-1109
- Paoletti, L., & Blakemore, R. 1986. Hydroxamate production by Aquaspirillum magnetotacticum. Journal of Bacteriology. Vol 167.1:73-76.
- Ricci, J. C., Woodford, B. J., Kirschvink, J. L., Hoffman, M. R. 1991. Alteration of the Magnetic Properties of Aquaspirillum magnetotacticum by a Pulse Magnetization Technique. Applied and Environmental Microbioloy. Vol 57.1:3248-3254.
Author
Page authored by Susan Jarosz and Megan Hull, students of Prof.Jay Lennon at Michigan State University.