Malaria resistance as a result of genetic mutation: Difference between revisions
| (33 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
='''Introduction'''= | ='''Introduction'''= | ||
==<u>Malaria</u>== | ==<u>Malaria</u>== | ||
Malaria is a life-threatening disease spread by the bite of an infected female Anopheles mosquito, which is considered a primary vector of the illness. That is why such a mosquito is also known as the Malaria Mosquito. The infection is caused by plasmodium, a parasitic protozoans sporozoan subclass Coccidia genus. Plasmodium infects red blood cells across various animals worldwide, such as mammals, birds, and reptiles. However, out of more than two hundred Plasmodium species, only four are responsible for malaria in humans - Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi. The last has been recently documented, causing infection in the regions of Southeast Asia. Nevertheless, malaria is most common in the tropical and subtropical regions, where the climate is dry with less precipitation: South and Southeast Asia, Sub-Saharan Africa, being the home of more than 90% of malaria cases globally. Malaria parasites affect the host by attacking the red blood cells to invade the immune system and remodel erythrocytes for their own use. The enzyme plasmepsin V in the malaria parasite can export hundreds of proteins that remodel the host's red blood cell, eventually annihilating it. The released parasite proteins destroy the oxygen-carrying hemoglobin and deform the cell surface. That, in turn, causes erythrocytes to stick to blood vessels and prevents the cells from circulation freely. The parasite uses this strategy of invading the red blood cells to hide from the host immune system, as white blood cells do not attack erythrocytes unless they are someone else's. | Malaria is a life-threatening disease spread by the bite of an infected female ''Anopheles'' mosquito, which is considered a primary vector of the illness. That is why such a mosquito is also known as the Malaria Mosquito.The infection is caused by plasmodium, a parasitic protozoans sporozoan subclass Coccidia genus.<ref>[“Plasmodium | Protozoan Genus.” n.d. Encyclopedia Britannica. https://www.britannica.com/science/Plasmodium-protozoan-genus.]</ref> Plasmodium infects red blood cells across various animals worldwide, such as mammals, birds, and reptiles. However, out of more than two hundred Plasmodium species, only four are responsible for malaria in humans - ''Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale,'' and ''Plasmodium knowlesi.'' The last has been recently documented, causing infection in the regions of Southeast Asia.<ref>[“Malaria Parasites.” 2015. Malaria Site. February 25, 2015. https://www.malariasite.com/malaria-parasites/.]</ref> Nevertheless, malaria is most common in the tropical and subtropical regions, where the climate is dry with less precipitation: South and Southeast Asia, Sub-Saharan Africa, being the home of more than 90% of malaria cases globally.<ref>[“Malaria-Risk Countries: Where It’s Most Common.” n.d. Mosquito Squad. https://www.mosquitosquad.com/blog/2019/december/malaria-risk-countries-where-it-s-most-common/.]</ref> Malaria parasites affect the host by attacking the red blood cells to invade the immune system and remodel erythrocytes for their own use. The enzyme ''plasmepsin V'' in the malaria parasite can export hundreds of proteins that remodel the host's red blood cell, eventually annihilating it. The released parasite proteins destroy the oxygen-carrying hemoglobin and deform the cell surface. That, in turn, causes erythrocytes to stick to blood vessels and prevents the cells from circulation freely. The parasite uses this strategy of invading the red blood cells to hide from the host immune system, as white blood cells do not attack erythrocytes unless they are someone else's.<ref>[“Molecule Allows Malaria Parasite to Commandeer Red Blood Cells.” 2010. HHMI.org. 2010. https://www.hhmi.org/news/molecule-allows-malaria-parasite-commandeer-red-blood-cells.]</ref> | ||
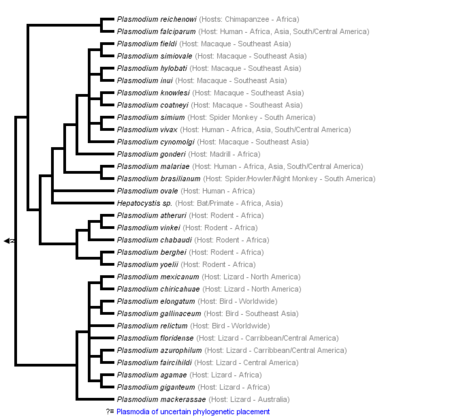
[[Image:KaranWikiImage.png|thumb|450px|left|'''Figure 1:''' Phylogeny of genus Plasmodium based on Escalante et al., 1995; Perkins and Schall, 2002; Vargas-Serrato et al., 2003; Martinsen et al., 2008 (From Tree of Life Web Project).[ | [[Image:KaranWikiImage.png|thumb|450px|left|'''Figure 1:''' Phylogeny of genus Plasmodium based on Escalante et al., 1995; Perkins and Schall, 2002; Vargas-Serrato et al., 2003; Martinsen et al., 2008 (From Tree of Life Web Project).<ref>[“Malaria Parasites.” 2015. Malaria Site. February 25, 2015. https://www.malariasite.com/malaria-parasites//.]</ref>.]] | ||
==<u>Sickle-cell Anemia</u>== | ==<u>Sickle-cell Anemia</u>== | ||
Sickle-cell anemia is an inherited disorder that affects red blood cells. Erythrocytes contain a red protein molecule hemoglobin that carries iron, which holds oxygen, thereby transporting the supply of oxygen throughout the body vessels. Typically, red blood cells tend to be round and flexible, which allows them to bend to move freely even through the narrowest blood vessels. However, individuals affected by sickle cell anemia have a mutation in the hemoglobin gene, which happens on the sixth codon of the beta chain of hemoglobin. The sequence of bases CTC is changed to CAC. Such variation in the gene does not code for the Glutamic acid anymore but instead for Valine. A change in hemoglobin gene information causes the hemoglobin to form strands inside the erythrocyte, making them inflexible and giving them a symbolic sickle-like shape. Such altered versions of red blood cells can cause blockages in the section of blood vessels, leading to sickle cell crisis due to the inability of red blood cells to supply all tissues and organs with oxygen. For disease expression, either two copies of sickle hemoglobin (Hb S) or one in addition to the normal beta-globin gene (Hb N) are required. | Sickle-cell anemia is an inherited disorder that affects red blood cells. Erythrocytes contain a red protein molecule hemoglobin that carries iron, which holds oxygen, thereby transporting the supply of oxygen throughout the body vessels. Typically, red blood cells tend to be round and flexible, which allows them to bend to move freely even through the narrowest blood vessels. However, individuals affected by sickle cell anemia have a mutation in the hemoglobin gene, which happens on the sixth codon of the beta chain of hemoglobin. The sequence of bases ''CTC'' is changed to ''CAC.'' Such variation in the gene does not code for the Glutamic acid anymore but instead for Valine.<ref>[“Sickle-Cell Anemia: Example of a ‘Beneficial Mutation’?” 2010. Creation Science Association of British Columbia. January 5, 2010. https://creationbc.org/index.php/sickle-cell-anemia-example-of-a-beneficial-mutation/.]</ref> A change in hemoglobin gene information causes the hemoglobin to form strands inside the erythrocyte, making them inflexible and giving them a symbolic sickle-like shape. Such altered versions of red blood cells can cause blockages in the section of blood vessels, leading to sickle cell crisis due to the inability of red blood cells to supply all tissues and organs with oxygen. For disease expression, either two copies of sickle hemoglobin (Hb S) or one in addition to the normal beta-globin gene (Hb N) are required. | ||
==<u>Relationship between malaria endemicity and frequency of mutated hemoglobin gene</u>== | ==<u>Relationship between malaria endemicity and frequency of mutated hemoglobin gene</u>== | ||
There is geographical support for a correlation between the high frequencies of the hemoglobin gene responsible for sickle cell disease and resistance against malaria by the heterozygous carrier of the beta-globin gene. Such correlation is the strongest in Africa, where Hb S allele frequencies and the level of malaria endemicity showed the highest numbers. <ref>[https:// | There is geographical support for a correlation between the high frequencies of the hemoglobin gene responsible for sickle cell disease and resistance against malaria by the heterozygous carrier of the beta-globin gene. Such correlation is the strongest in Africa, where Hb S allele frequencies and the level of malaria endemicity showed the highest numbers. <ref>[Piel, Frédéric B., Anand P. Patil, Rosalind E. Howes, Oscar A. Nyangiri, Peter W. Gething, Thomas N. Williams, David J. Weatherall, and Simon I. Hay. 2010. “Global Distribution of the Sickle Cell Gene and Geographical Confirmation of the Malaria Hypothesis.” Nature Communications 1 (1). https://doi.org/10.1038/ncomms1104.]</ref> Another study conducted in one of the hospitals in Cameroon concluded that individuals with expressed sickle cell anemia had a lower mortality rate than individuals with unmutated beta chain hemoglobin. <ref>[Eleonore, Ngo Linwa Esther, Samuel Nambile Cumber, Eposse Ekoube Charlotte, Esuh Esong Lucas, Mandeng Ma Linwa Edgar, Claude Ngwayu Nkfusai, Meh Martin Geh, et al. 2020. “Malaria in Patients with Sickle Cell Anaemia: Burden, Risk Factors and Outcome at the Laquintinie Hospital, Cameroon.” BMC Infectious Diseases 20 (1). https://doi.org/10.1186/s12879-019-4757-x.]</ref> | ||
<br> | <br><br> | ||
<br> | |||
='''Genetics'''= | |||
==<u>Mechanism of protection</u>== | ==<u>Mechanism of protection</u>== | ||
It is a question of ongoing research on how living organisms such as animals and plants can develop protection mechanisms against parasites. In the case of invasion of malaria plasmodium into the red blood cells, it is a multi-step process, which involves interactions on genetic, biochemical, and immunological levels. | It is a question of ongoing research on how living organisms such as animals and plants can develop protection mechanisms against parasites. In the case of invasion of malaria plasmodium into the red blood cells, it is a multi-step process, which involves interactions on genetic, biochemical, and immunological levels. | ||
== | ===''Molecular mechanism''=== | ||
It turns out that one of the protective effects which prevented the development of parasites inside the red blood cells was haeme oxygenase-1, a stress-responsive enzyme whose expression is strongly induced by sickle hemoglobin, which increases the rate of free heme. HO-1 expression appears mediated by the transcription factor NrF2. The enzyme converts the protoporphyrin IX ring of heme, emitted by Hb S, into biliverdin, leading to the release of iron and carbon monoxide production. The HO-1 gene (HMOX-1) is frequently activated under cellular stress conditions. The end-product carbon monoxide gas level is not high enough to poison the host itself but enough to be lethal for the plasmodium parasite. Therefore, a heterozygous host for a sickle cell gene is prevented from experiencing malaria symptoms since the parasite could not enter the red blood cells and reproduce. | It turns out that one of the protective effects which prevented the development of parasites inside the red blood cells was ''haeme oxygenase-1,'' a stress-responsive enzyme whose expression is strongly induced by sickle hemoglobin, which increases the rate of free heme. HO-1 expression appears mediated by the transcription factor ''NrF2.'' The enzyme converts the protoporphyrin IX ring of heme, emitted by Hb S, into biliverdin, leading to the release of iron and carbon monoxide production. The ''HO-1 gene (HMOX-1)'' is frequently activated under cellular stress conditions. The end-product carbon monoxide gas level is not high enough to poison the host itself but enough to be lethal for the plasmodium parasite. Therefore, a heterozygous host for a sickle cell gene is prevented from experiencing malaria symptoms since the parasite could not enter the red blood cells and reproduce.<ref name=" Eridani ">[Eridani, Sandro. 2011. “Sickle Cell Protection from Malaria: A Review.” Hematology Reports (Formerly Hematology Reviews) 3 (3). https://doi.org/10.4081/hr.2011.e24.]</ref> | ||
Enhanced phagocytosis of ring-parasitized mutant red blood cells may indicate a mechanism of protection in non-immune individuals affected by sickle trait. Since Hb S tends to be more unstable than the normal Hb N, it clusters red cell membrane proteins, which leads to the removal of mutant erythrocytes by phagocytic cells. | ===''Phagocytosis''=== | ||
== | Enhanced phagocytosis of ring-parasitized mutant red blood cells may indicate a mechanism of protection in non-immune individuals affected by sickle trait. Since Hb S tends to be more unstable than the normal Hb N, it clusters red cell membrane proteins, which leads to the removal of mutant erythrocytes by phagocytic cells.<ref name=" Eridani ">[Eridani, Sandro. 2011. “Sickle Cell Protection from Malaria: A Review.” Hematology Reports (Formerly Hematology Reviews) 3 (3). https://doi.org/10.4081/hr.2011.e24.]</ref> | ||
The multiple-hit system makes the free heme synergize with a cytotoxic agonist CD8+ T pathogenic cells, which combination might trigger pathogenic cerebral malaria. However, as stated earlier, Hb S represses the formation of free heme, but it also opposes the activation of | |||
===''Immune mechanism''=== | |||
The multiple-hit system makes the free heme synergize with a cytotoxic agonist ''</b> CD8<sup>+</sup>T'' pathogenic cells, which combination might trigger pathogenic cerebral malaria. However, as stated earlier, Hb S represses the formation of free heme, but it also opposes the activation of ''</b> CD8<sup>+</sup>T'' cells. Therefore, such a form of immune regulation might explain the protective effect of the sickle cell trait against the disease.<ref name=" Eridani ">[Eridani, Sandro. 2011. “Sickle Cell Protection from Malaria: A Review.” Hematology Reports (Formerly Hematology Reviews) 3 (3). https://doi.org/10.4081/hr.2011.e24.]</ref> | |||
='''Microbiome'''= | |||
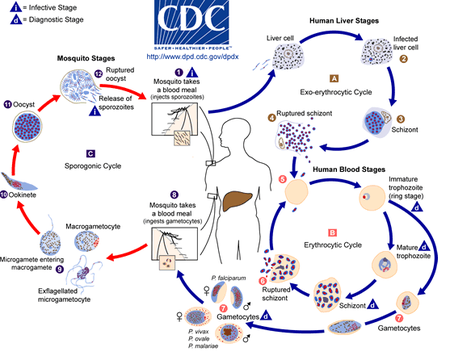
[[Image:plasmodiumlifecycle.png|thumb|450px|left|'''Figure 2:''' The malaria parasite life cycle.<ref>[CDC. 2019. “CDC - DPDx - Malaria.” Centers for Disease Control and Prevention. 2019. https://www.cdc.gov/dpdx/malaria/index.html.]</ref>]] | [[Image:plasmodiumlifecycle.png|thumb|450px|left|'''Figure 2:''' The malaria parasite life cycle.<ref>[CDC. 2019. “CDC - DPDx - Malaria.” Centers for Disease Control and Prevention. 2019. https://www.cdc.gov/dpdx/malaria/index.html.]</ref>]] | ||
==<u>Life cycle of a parasite</u>== | ==<u>Life cycle of a parasite</u>== | ||
Initially, Plasmodium is in a stage of development called a sporozoite in the infected female mosquito's salivary gland. When the mosquito pierces a person's skin, sporozoites, which remind a worm-like shape, spill out of the mosquito's saliva and get into the bloodstream. Within the next few minutes, the parasites reach a person's liver, starting asexual reproduction, also known as schizogony. Over the next couple of weeks, the parasite matures from sporozoite to merozoite while destroying the liver host's cells. This stage is called exoerythrocytic because it happens outside of the red blood cells and is typically asymptomatic. The merozoites are then released into the bloodstream, where they bind on the surface receptors of the red blood cells and invade them. Inside the red blood cells, the merozoite undergoes asexual reproduction once again for two or three days. Such phase is known as erythrocytic because it happens inside the red blood cells. The parasite grows by digesting hemoglobin inside the cell. A parasite is now called a schizont, which undergoes mitosis and differentiates into many merozoites. The parasite can be released into the blood by bursting the red blood cell. Some merozoites undergo gametogony, giving rise to gametocytes that can be male or female. These gametocytes remain in the red blood cells and, after some time, can be sucked up by another Anopheles mosquito with blood from the infected person. Gametocytes can then reach a mosquito's gut, mature, and form a zygote. This cycle phase is called sporogony, which is sexual reproduction compared to schizogony, asexual reproduction. The zygote develops further and becomes ookinete, then oocyst, which ruptures in the mosquito's gut. Thousands of sporozoites are released, which go into the mosquito's salivary gland to repeat the cycle. | Initially, Plasmodium is in a stage of development called a sporozoite in the infected female mosquito's salivary gland. When the mosquito pierces a person's skin, sporozoites, which remind a worm-like shape, spill out of the mosquito's saliva and get into the bloodstream. Within the next few minutes, the parasites reach a person's liver, starting asexual reproduction, also known as schizogony.<ref name=" kito ">[N, Supriya. 2020. “Life Cycle of Plasmodium Species - Asexual Phase, Sexual Phase & Summary.” Biology Reader. December 29, 2020. https://biologyreader.com/life-cycle-of-plasmodium-species.html.]</ref>Over the next couple of weeks, the parasite matures from sporozoite to merozoite while destroying the liver host's cells. This stage is called exoerythrocytic because it happens outside of the red blood cells and is typically asymptomatic. The merozoites are then released into the bloodstream, where they bind on the surface receptors of the red blood cells and invade them. Inside the red blood cells, the merozoite undergoes asexual reproduction once again for two or three days. Such phase is known as erythrocytic because it happens inside the red blood cells. The parasite grows by digesting hemoglobin inside the cell. A parasite is now called a schizont, which undergoes mitosis and differentiates into many merozoites. The parasite can be released into the blood by bursting the red blood cell. Some merozoites undergo gametogony, giving rise to gametocytes that can be male or female. These gametocytes remain in the red blood cells and, after some time, can be sucked up by another Anopheles mosquito with blood from the infected person. Gametocytes can then reach a mosquito's gut, mature, and form a zygote. This cycle phase is called sporogony, which is sexual reproduction compared to schizogony, asexual reproduction. The zygote develops further and becomes ookinete, then oocyst, which ruptures in the mosquito's gut. Thousands of sporozoites are released, which go into the mosquito's salivary gland to repeat the cycle.<ref name=" kito ">[N, Supriya. 2020. “Life Cycle of Plasmodium Species - Asexual Phase, Sexual Phase & Summary.” Biology Reader. December 29, 2020. https://biologyreader.com/life-cycle-of-plasmodium-species.html.]</ref> | ||
<br> | |||
=<u>Host's response to the infection with parasite</u>= | ==<u>Host's response to the infection with parasite</u>== | ||
The release of tumor necrosis factor-alpha ( | The release of tumor necrosis factor-alpha ''(TNFα)'' and other inflammatory cytokines causes shivering and chills, followed by an abnormally high fever. Such symptoms happen in paroxysms, known as short bursts, which correspond to the rupture of the infected red blood cells. This happens in the waves of reproductive cycles unique for each Plasmodium species. ''P. malariae'' fever happens every 72 hours, also defined as quartan fever. For ''P. vivax'' and ''P. ovale,'' fever happens every 48 hours, which is called tertian fevers. ''P. knowlesi'' causes fever every 24 hours, and ''P. falciparum'' does not have a fever paroxysms, which means that the time between fevers can vary. Such type of fever is known as malignant tertian fever.<ref name=" kito ">[N, Supriya. 2020. “Life Cycle of Plasmodium Species - Asexual Phase, Sexual Phase & Summary.” Biology Reader. December 29, 2020. https://biologyreader.com/life-cycle-of-plasmodium-species.html.]</ref> In addition to fever, the destruction of red blood cells, also known as hemolytic anemia, causes symptoms like fatigue and headaches.<ref>[Mayo Clinic. 2021. “Malaria - Symptoms and Causes.” Mayo Clinic. February 3, 2021. https://www.mayoclinic.org/diseases-conditions/malaria/symptoms-causes/syc-20351184.]</ref> | ||
='''Conclusion'''= | |||
Mutations happen randomly in the genome of every generation, most of which are not beneficial. However, when a new mutation occurs under positive natural selection, meaning that it is beneficial, it is more likely to survive in the population. Such a case can be seen in malaria, which has been one of the major infectious diseases in terms of public health. The sickle trait is relevant to the idea of natural selection and has been beneficial in both survival and reproductive success. Even though sickle cell anemia is damaging in one way or another, it has been inherited through many generations, and the heterozygous frequency of mutant hemoglobin genome spread over the area of the Malarian belt. The relationship between malaria and the mutation of the hemoglobin genome points us to the dramatic forces of natural selection, reshaping the human genome. | |||
='''References'''= | |||
<references /> | <references /> | ||
Latest revision as of 01:47, 10 December 2021
Introduction
Malaria
Malaria is a life-threatening disease spread by the bite of an infected female Anopheles mosquito, which is considered a primary vector of the illness. That is why such a mosquito is also known as the Malaria Mosquito.The infection is caused by plasmodium, a parasitic protozoans sporozoan subclass Coccidia genus.[1] Plasmodium infects red blood cells across various animals worldwide, such as mammals, birds, and reptiles. However, out of more than two hundred Plasmodium species, only four are responsible for malaria in humans - Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi. The last has been recently documented, causing infection in the regions of Southeast Asia.[2] Nevertheless, malaria is most common in the tropical and subtropical regions, where the climate is dry with less precipitation: South and Southeast Asia, Sub-Saharan Africa, being the home of more than 90% of malaria cases globally.[3] Malaria parasites affect the host by attacking the red blood cells to invade the immune system and remodel erythrocytes for their own use. The enzyme plasmepsin V in the malaria parasite can export hundreds of proteins that remodel the host's red blood cell, eventually annihilating it. The released parasite proteins destroy the oxygen-carrying hemoglobin and deform the cell surface. That, in turn, causes erythrocytes to stick to blood vessels and prevents the cells from circulation freely. The parasite uses this strategy of invading the red blood cells to hide from the host immune system, as white blood cells do not attack erythrocytes unless they are someone else's.[4]
Sickle-cell Anemia
Sickle-cell anemia is an inherited disorder that affects red blood cells. Erythrocytes contain a red protein molecule hemoglobin that carries iron, which holds oxygen, thereby transporting the supply of oxygen throughout the body vessels. Typically, red blood cells tend to be round and flexible, which allows them to bend to move freely even through the narrowest blood vessels. However, individuals affected by sickle cell anemia have a mutation in the hemoglobin gene, which happens on the sixth codon of the beta chain of hemoglobin. The sequence of bases CTC is changed to CAC. Such variation in the gene does not code for the Glutamic acid anymore but instead for Valine.[6] A change in hemoglobin gene information causes the hemoglobin to form strands inside the erythrocyte, making them inflexible and giving them a symbolic sickle-like shape. Such altered versions of red blood cells can cause blockages in the section of blood vessels, leading to sickle cell crisis due to the inability of red blood cells to supply all tissues and organs with oxygen. For disease expression, either two copies of sickle hemoglobin (Hb S) or one in addition to the normal beta-globin gene (Hb N) are required.
Relationship between malaria endemicity and frequency of mutated hemoglobin gene
There is geographical support for a correlation between the high frequencies of the hemoglobin gene responsible for sickle cell disease and resistance against malaria by the heterozygous carrier of the beta-globin gene. Such correlation is the strongest in Africa, where Hb S allele frequencies and the level of malaria endemicity showed the highest numbers. [7] Another study conducted in one of the hospitals in Cameroon concluded that individuals with expressed sickle cell anemia had a lower mortality rate than individuals with unmutated beta chain hemoglobin. [8]
Genetics
Mechanism of protection
It is a question of ongoing research on how living organisms such as animals and plants can develop protection mechanisms against parasites. In the case of invasion of malaria plasmodium into the red blood cells, it is a multi-step process, which involves interactions on genetic, biochemical, and immunological levels.
Molecular mechanism
It turns out that one of the protective effects which prevented the development of parasites inside the red blood cells was haeme oxygenase-1, a stress-responsive enzyme whose expression is strongly induced by sickle hemoglobin, which increases the rate of free heme. HO-1 expression appears mediated by the transcription factor NrF2. The enzyme converts the protoporphyrin IX ring of heme, emitted by Hb S, into biliverdin, leading to the release of iron and carbon monoxide production. The HO-1 gene (HMOX-1) is frequently activated under cellular stress conditions. The end-product carbon monoxide gas level is not high enough to poison the host itself but enough to be lethal for the plasmodium parasite. Therefore, a heterozygous host for a sickle cell gene is prevented from experiencing malaria symptoms since the parasite could not enter the red blood cells and reproduce.[9]
Phagocytosis
Enhanced phagocytosis of ring-parasitized mutant red blood cells may indicate a mechanism of protection in non-immune individuals affected by sickle trait. Since Hb S tends to be more unstable than the normal Hb N, it clusters red cell membrane proteins, which leads to the removal of mutant erythrocytes by phagocytic cells.[9]
Immune mechanism
The multiple-hit system makes the free heme synergize with a cytotoxic agonist CD8+T pathogenic cells, which combination might trigger pathogenic cerebral malaria. However, as stated earlier, Hb S represses the formation of free heme, but it also opposes the activation of CD8+T cells. Therefore, such a form of immune regulation might explain the protective effect of the sickle cell trait against the disease.[9]
Microbiome
Life cycle of a parasite
Initially, Plasmodium is in a stage of development called a sporozoite in the infected female mosquito's salivary gland. When the mosquito pierces a person's skin, sporozoites, which remind a worm-like shape, spill out of the mosquito's saliva and get into the bloodstream. Within the next few minutes, the parasites reach a person's liver, starting asexual reproduction, also known as schizogony.[11]Over the next couple of weeks, the parasite matures from sporozoite to merozoite while destroying the liver host's cells. This stage is called exoerythrocytic because it happens outside of the red blood cells and is typically asymptomatic. The merozoites are then released into the bloodstream, where they bind on the surface receptors of the red blood cells and invade them. Inside the red blood cells, the merozoite undergoes asexual reproduction once again for two or three days. Such phase is known as erythrocytic because it happens inside the red blood cells. The parasite grows by digesting hemoglobin inside the cell. A parasite is now called a schizont, which undergoes mitosis and differentiates into many merozoites. The parasite can be released into the blood by bursting the red blood cell. Some merozoites undergo gametogony, giving rise to gametocytes that can be male or female. These gametocytes remain in the red blood cells and, after some time, can be sucked up by another Anopheles mosquito with blood from the infected person. Gametocytes can then reach a mosquito's gut, mature, and form a zygote. This cycle phase is called sporogony, which is sexual reproduction compared to schizogony, asexual reproduction. The zygote develops further and becomes ookinete, then oocyst, which ruptures in the mosquito's gut. Thousands of sporozoites are released, which go into the mosquito's salivary gland to repeat the cycle.[11]
Host's response to the infection with parasite
The release of tumor necrosis factor-alpha (TNFα) and other inflammatory cytokines causes shivering and chills, followed by an abnormally high fever. Such symptoms happen in paroxysms, known as short bursts, which correspond to the rupture of the infected red blood cells. This happens in the waves of reproductive cycles unique for each Plasmodium species. P. malariae fever happens every 72 hours, also defined as quartan fever. For P. vivax and P. ovale, fever happens every 48 hours, which is called tertian fevers. P. knowlesi causes fever every 24 hours, and P. falciparum does not have a fever paroxysms, which means that the time between fevers can vary. Such type of fever is known as malignant tertian fever.[11] In addition to fever, the destruction of red blood cells, also known as hemolytic anemia, causes symptoms like fatigue and headaches.[12]
Conclusion
Mutations happen randomly in the genome of every generation, most of which are not beneficial. However, when a new mutation occurs under positive natural selection, meaning that it is beneficial, it is more likely to survive in the population. Such a case can be seen in malaria, which has been one of the major infectious diseases in terms of public health. The sickle trait is relevant to the idea of natural selection and has been beneficial in both survival and reproductive success. Even though sickle cell anemia is damaging in one way or another, it has been inherited through many generations, and the heterozygous frequency of mutant hemoglobin genome spread over the area of the Malarian belt. The relationship between malaria and the mutation of the hemoglobin genome points us to the dramatic forces of natural selection, reshaping the human genome.
References
- ↑ [“Plasmodium | Protozoan Genus.” n.d. Encyclopedia Britannica. https://www.britannica.com/science/Plasmodium-protozoan-genus.]
- ↑ [“Malaria Parasites.” 2015. Malaria Site. February 25, 2015. https://www.malariasite.com/malaria-parasites/.]
- ↑ [“Malaria-Risk Countries: Where It’s Most Common.” n.d. Mosquito Squad. https://www.mosquitosquad.com/blog/2019/december/malaria-risk-countries-where-it-s-most-common/.]
- ↑ [“Molecule Allows Malaria Parasite to Commandeer Red Blood Cells.” 2010. HHMI.org. 2010. https://www.hhmi.org/news/molecule-allows-malaria-parasite-commandeer-red-blood-cells.]
- ↑ [“Malaria Parasites.” 2015. Malaria Site. February 25, 2015. https://www.malariasite.com/malaria-parasites//.]
- ↑ [“Sickle-Cell Anemia: Example of a ‘Beneficial Mutation’?” 2010. Creation Science Association of British Columbia. January 5, 2010. https://creationbc.org/index.php/sickle-cell-anemia-example-of-a-beneficial-mutation/.]
- ↑ [Piel, Frédéric B., Anand P. Patil, Rosalind E. Howes, Oscar A. Nyangiri, Peter W. Gething, Thomas N. Williams, David J. Weatherall, and Simon I. Hay. 2010. “Global Distribution of the Sickle Cell Gene and Geographical Confirmation of the Malaria Hypothesis.” Nature Communications 1 (1). https://doi.org/10.1038/ncomms1104.]
- ↑ [Eleonore, Ngo Linwa Esther, Samuel Nambile Cumber, Eposse Ekoube Charlotte, Esuh Esong Lucas, Mandeng Ma Linwa Edgar, Claude Ngwayu Nkfusai, Meh Martin Geh, et al. 2020. “Malaria in Patients with Sickle Cell Anaemia: Burden, Risk Factors and Outcome at the Laquintinie Hospital, Cameroon.” BMC Infectious Diseases 20 (1). https://doi.org/10.1186/s12879-019-4757-x.]
- ↑ 9.0 9.1 9.2 [Eridani, Sandro. 2011. “Sickle Cell Protection from Malaria: A Review.” Hematology Reports (Formerly Hematology Reviews) 3 (3). https://doi.org/10.4081/hr.2011.e24.]
- ↑ [CDC. 2019. “CDC - DPDx - Malaria.” Centers for Disease Control and Prevention. 2019. https://www.cdc.gov/dpdx/malaria/index.html.]
- ↑ 11.0 11.1 11.2 [N, Supriya. 2020. “Life Cycle of Plasmodium Species - Asexual Phase, Sexual Phase & Summary.” Biology Reader. December 29, 2020. https://biologyreader.com/life-cycle-of-plasmodium-species.html.]
- ↑ [Mayo Clinic. 2021. “Malaria - Symptoms and Causes.” Mayo Clinic. February 3, 2021. https://www.mayoclinic.org/diseases-conditions/malaria/symptoms-causes/syc-20351184.]
Edited by [Karan Mehta], student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2021, Kenyon College.
