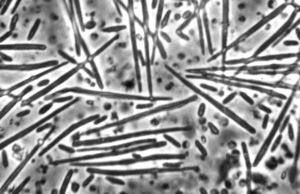
Metschnikowia bicuspidata
Description and Significance
Metschnikowia is a genus in the Kingdom Fungi (Naumov, 2011). Metschnikowia are single-celled (i.e., yeast) parasites of freshwater animals, usually crustaceans, and particularly of zooplankton in the genus Daphnia (ibid.).
The organism has two morphological forms: round vegetative cells and single-celled needle-shaped propagative spores (Naumov, 2011). M. bicuspidata uses energy from the host Daphnia to produce tens of thousands of identical haploid spores. These increase in number until the Daphnia is killed and its carapace ruptures, introducing tens of thousands of new spores into the water column.
Daphnia and M. bicuspidata are used in ecology and evolutionary biology to study ecological phenomena in aquatic ecosystems. M. bicuspidata is used to study host-parasite dynamics and the effects of parasitism on host community evolution.
M. bicuspidata has been found to infect non-Daphnia animals, including several small crustaceans, prawns, and salmon (Moore and Strom, 2003). The prawn experienced changes in tissue coloration, edema, inflammation in muscles, swollen organs, necrotic lesions, and early death, among other symptoms (Chen et al., 2003 and 2007). They contained yeast at approximately 10(8) to 10(9) colony forming units per 100 mg of tissue (Chen et al., 2007). Salmon that were fed Artemia brine shrimp containing the fungus experienced increased mortality due to infections of M. bicuspidata (Moore and Strom, 2003).
Classification
Eukaryota, Fungi, Dikarya, Ascomycota, Saccharomycotina, Saccharomycetes, Saccharomycetales, Metschnikowiaceae, Metschnikowia (European Nucleotide Archive, accessed 21 April 2015)
There are three varieties in the species: M. bicuspidata var. bicuspidata, var. californica, and var. chathamia (Naumov, 2011).
Genome Structure
The genome of Metschnikowia bicuspidata NRRL YB-4993 is 16.06 Mbp long. Being a eukaryote, this parasite's DNA is linear. This genome of this species contains 1376 genes associated with cellular processes and signaling, 1088 genes associated with information storage and processing, 1237 genes associated with metabolism, and 877 genes that are poorly characterized.
26S statistics for M. bicuspidata var. bicuspidata show an average similarity between strains to be 99.3379%, while var. chathamia has an average similarity of 98.5696% between strains.
Cell Structure, Metabolism and Life Cycle
M. bicuspidata is a chemoorganoheterotroph, and gets its energy by parasitising the freshwater zooplankton Daphnia (Hall et al., 2009). The sharp needle-shaped cells suggest a puncture mechanism of entering the Daphnia body.
Life cycle: Needle-shaped cells (n) float in the water column and are eaten by Daphnia. In the Daphnia hemolymph, these cells (n) produce spherical vegetative cells (n) that undergo plasmogamy (n + n), followed shortly by karyogamy (2n). Some of these cells (2n) will undergo mitotic haploidization, producing more cells (n), while some will undergo meiotic haploidization, producing asci, containing needle-like ascospores (n). Ascospores penetrate the carapace of hosts, releasing spores (n) into the water column, where they can be eaten by future hosts (life cycle information from Naumov, 2011).
The haploid ascospores are the virulent form of M. bicuspidata (Naumov, 2011).
M. bicuspidata can survive at temperatures of 9-27 degrees C, salinity of 270 ppt, and NaCl concentrations of 0-180 ppt (Moore and Strom, 2003). (For comparison, sea water salinity is approximately 35 ppt).
M. bicuspidata variants can be determined by their metabolic activities. M. bicuspidata var. californica has the ability to assimilate methyl-α-D-glucoside, D-gluconate and s-Keto-D-gluconate. M. bicuspidata var. chathamia can assimiliate methyl-α-D-glucoside, but cannot assimilate D-gluconate or 2-keto-D-gluconate. M. bicuspidata var. bicuspidata is unable to assimilate D-glucoside.
Ecology and Pathogenesis
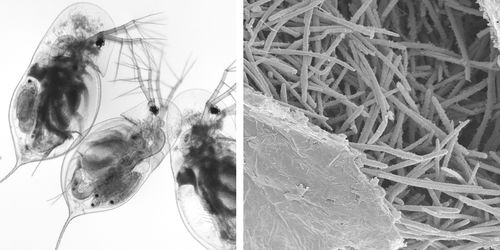
Exposure of host Daphnia to parasitic M. bicuspidata occurs during feeding. Daphnia eat algae in the water column and feed indiscriminately on whatever floating particles fit into their mouths. During feeding, M. bicuspidata spores enter the Daphnia mouth, travel along the gut, and puncture the gut wall. Infection occurs when a spore punctures the wall, enters the hemolymph, and begin reproducing (Ebert et al., 2000).
M. bicuspidata kills the host D. magna in 7-25 days (average = 17.5 days); a healthy D. magna typically lives 40-80 days (Ebert et al., 2000).
Virulence depends on the quality of food eaten by the Daphnia (Hall et al., 2009). Daphnia die more quickly from infection when they are fed more, better quality food than when they are fed very little or low-quality food (Hall et al., 2009).
Susceptibility to infection varies across host genotypes ( ). Infected D. magna exhibit reduced fecundity (Ebert et al., 2000). Upon death due to infection, Daphnia may yield 10,000 to 70,000 spores per individual (Penczykowski et al., 2014).
M. bicuspidata uses energy from the Daphnia body to reproduce (Hall et al., 2009).
Transmission is horizontal, meaning infection occurs within a host generation and is not transmitted from parent to offspring (Ebert et al., 2000).
References
[2] [Chen, SC., Chen, TH., Wang, PC., Chen, YC., Huang, JP., Lin, YD., Chaung, HC., Liaw, LL. "Metschnikowia bicuspidata and Enterococcus faecium co-infection in the giant freshwater prawn Macrobrachium rosenbergii." Diseases of Aquatic Organisms. 2003. Volume 55(2). pp. 161-167.]
[3] [Chen, SC., Chen, YC., Kwang, JM., Manopo, I., Wang, PC., Chaung, HC., Liaw, LL., Chiu, SH. "Metschnikowia bicuspidata dominates in Taiwanese cold-weather yeast infections of a Macrobrachium rosenbergii." Diseases of Aquatic Organisms. 2007. Volume 75(3). pp. 191-199.]
[4] [Ebert, D., Lipsitch, M., Mangin, K. L. "The Effect of Parasites on Host Population Density and Extinction: Experimental Epidemiology with Daphnia and Six Microparasites." The American Naturalist. 2000. Volume 156(5). pp. 459-477.]
[5] [Hall, S. R., Simonis, J. L., Nisbet, R. M., Tessier, A. J., Cáceres, C. E. "Resource Ecology of Virulence in a Planktonic Host‐Parasite System: An Explanation Using Dynamic Energy Budgets." The American Naturalist. 2009. Volume 174(2). pp. 149-162.]
[6] [Moore, M., and Strom, M. "Infection and mortality by the yeast Metschnikowia bicuspidata var. bicuspidata in chinook salmon fed live adult brine shrimp (Artemia franciscana)." Aquaculture. 2003. Volume 220(1-4). pp. 43-57.]
[7] [Naumov, G. I. "Molecular and genetic differentiation of small-spored species of the yeast genus Metschnikowia Kamienski." Microbiology. 2011. Volume 80(2). pp. 135-142.]
Penczykowski, R. M., Lemanski, B. C. P., Sieg, R. D., Hall, S. R., Ochs, J. H., Kubanek, J., Duffy, M. A. "Poor resource quality lowers transmission potential by changing foraging behavior." Functional Ecology. 2014. Volume 28. pp. 1245-1255.
Author
Page authored by Katie Griebel and Jacob Gelarden, students of Prof. Jay Lennon at Indiana University.