Microbial Ecology of Subglacial Environments: Difference between revisions
| Line 5: | Line 5: | ||
The defining characteristics of subglacial environments include the complete lack of light<ref name = Boetius et al. 2015> [https://www.nature.com/articles/nrmicro3522 Boetius et al.: Microbial ecology of the cryosphere:sea ice and glacial habitats. Nature Reviews 2015, v. 13, p. 677-690.]</ref>, largely anoxic conditions<ref name = Wadham et al. 2004>[https://www.sciencedirect.com/science/article/pii/S0012821X03006836?casa_token=WfsXOx4oSqQAAAAA:WHqhHaedE1sq3ip6lTWcxdI-u-qBva_FgVPAXiX_U3ajjmgxg104iJ4g9gaCjmUS9xxnW2A1Fm4 Wadhma et al.: Stable isotope evidence for microbial sulphate reduction at the bed of a polythermal high Arctic glacier. Earth and Planetary Science Letters 2004, v. 219, issues 3-4, p. 341-355.]</ref><ref name = Wynn et al. 2005>[https://link.springer.com/article/10.1007/s10533-005-3832-0 Wynn et al.: Chemical and isotopic switching within the subglacial environment of a High Arctic glacier. Biogeochemistry 2006, v. 78, p. 173-193.]</ref>, and low temperatures (around 0⁰C )<ref name = Hamilton et al. 2013>[https://www.nature.com/articles/ismej201331 Hamilton et al.: Molecular evidence for an active endogenous microbiome beneath glacial ice. The ISMEJ 2013, v.7, p. 1402-1412.]</ref>. Despite these common characteristics, subglacial environments are diverse in their environmental attributes. This partly derives from the diversity of Earth’s cryosphere. As suggested by the name, subglacial environments are found beneath glaciers, both alpine and outlet, but also below Earth’s massive ice sheets – the Antarctic and Greenlandic. These differing environments, while seemingly similar, are quite distinct and require their own fields of study. Glaciers only need be tens of meters thick, while ice sheets are kilometers thick. | The defining characteristics of subglacial environments include the complete lack of light<ref name = "Boetius et al. 2015"> [https://www.nature.com/articles/nrmicro3522 Boetius et al.: Microbial ecology of the cryosphere:sea ice and glacial habitats. Nature Reviews 2015, v. 13, p. 677-690.]</ref>, largely anoxic conditions<ref name = Wadham et al. 2004>[https://www.sciencedirect.com/science/article/pii/S0012821X03006836?casa_token=WfsXOx4oSqQAAAAA:WHqhHaedE1sq3ip6lTWcxdI-u-qBva_FgVPAXiX_U3ajjmgxg104iJ4g9gaCjmUS9xxnW2A1Fm4 Wadhma et al.: Stable isotope evidence for microbial sulphate reduction at the bed of a polythermal high Arctic glacier. Earth and Planetary Science Letters 2004, v. 219, issues 3-4, p. 341-355.]</ref><ref name = Wynn et al. 2005>[https://link.springer.com/article/10.1007/s10533-005-3832-0 Wynn et al.: Chemical and isotopic switching within the subglacial environment of a High Arctic glacier. Biogeochemistry 2006, v. 78, p. 173-193.]</ref>, and low temperatures (around 0⁰C )<ref name = "Hamilton et al. 2013">[https://www.nature.com/articles/ismej201331 Hamilton et al.: Molecular evidence for an active endogenous microbiome beneath glacial ice. The ISMEJ 2013, v.7, p. 1402-1412.]</ref>. Despite these common characteristics, subglacial environments are diverse in their environmental attributes. This partly derives from the diversity of Earth’s cryosphere. As suggested by the name, subglacial environments are found beneath glaciers, both alpine and outlet, but also below Earth’s massive ice sheets – the Antarctic and Greenlandic. These differing environments, while seemingly similar, are quite distinct and require their own fields of study. Glaciers only need be tens of meters thick, while ice sheets are kilometers thick. | ||
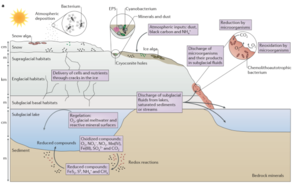
Although defined by the presence of solid water (ice), many subglacial environments also contain liquid water – a required component for all life, including microbes<ref name = Christner et al. 2008>[https://link.springer.com/chapter/10.1007/978-3-540-74335-4_4 Christner et al.: Bacteria in subglacial environments in R Margesin et al., eds., Pyschorphiles: from Biodiversity to Biotechnology 2008: Berlin, Springer, p. 51-71.]</ref>. Below the ice of warm and polythermal glaciers high pressures result in liquid water<ref name = Alley et al. 1997>[https://www.sciencedirect.com/science/article/pii/S0277379197000346?casa_token=w-FL-mXo9k0AAAAA:mxTm9WscBZrAVn4q5Rlauyf8ldg0wvSjS57QhrfsOZSUqzQ6xucWdmiUQT8SLwUa-q-i4MnYwMY Alley et al.: How glaciers entrain and transport basal sediment: physical constraints. Quaternary Science Reviews 1997, v. 16, p. 1017-1038.]</ref> (see Fig 2.). The amount and distribution of this water can vary from saturated sediments, to localized channels, to subglacial lakes<ref name = Priscu et al. 2008>[https://books.google.com/books?hl=en&lr=&id=ppUSDAAAQBAJ&oi=fnd&pg=PA119&dq=priscu+2008+antarctic+subglacial+water&ots=jnhL3plxwD&sig=BUPt9sRbfKz1niGDCursIrZSqCc#v=onepage&q=priscu%202008%20antarctic%20subglacial%20water&f=false Priscu et al.: Antarctic subglacial water: origin, evolution, and ecology in Vincent, W.F.., and Laybourn-Parry, J., eds., Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems 2008: Oxford, Oxford University Press.]</ref>. | Although defined by the presence of solid water (ice), many subglacial environments also contain liquid water – a required component for all life, including microbes<ref name = Christner et al. 2008>[https://link.springer.com/chapter/10.1007/978-3-540-74335-4_4 Christner et al.: Bacteria in subglacial environments in R Margesin et al., eds., Pyschorphiles: from Biodiversity to Biotechnology 2008: Berlin, Springer, p. 51-71.]</ref>. Below the ice of warm and polythermal glaciers high pressures result in liquid water<ref name = Alley et al. 1997>[https://www.sciencedirect.com/science/article/pii/S0277379197000346?casa_token=w-FL-mXo9k0AAAAA:mxTm9WscBZrAVn4q5Rlauyf8ldg0wvSjS57QhrfsOZSUqzQ6xucWdmiUQT8SLwUa-q-i4MnYwMY Alley et al.: How glaciers entrain and transport basal sediment: physical constraints. Quaternary Science Reviews 1997, v. 16, p. 1017-1038.]</ref> (see Fig 2.). The amount and distribution of this water can vary from saturated sediments, to localized channels, to subglacial lakes<ref name = Priscu et al. 2008>[https://books.google.com/books?hl=en&lr=&id=ppUSDAAAQBAJ&oi=fnd&pg=PA119&dq=priscu+2008+antarctic+subglacial+water&ots=jnhL3plxwD&sig=BUPt9sRbfKz1niGDCursIrZSqCc#v=onepage&q=priscu%202008%20antarctic%20subglacial%20water&f=false Priscu et al.: Antarctic subglacial water: origin, evolution, and ecology in Vincent, W.F.., and Laybourn-Parry, J., eds., Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems 2008: Oxford, Oxford University Press.]</ref>. | ||
[[Image:Boeitus_et_al._2015_Diagram.png|thumb|300px|top|Fig. 2 Structure of subglacial environments, within the greater glacial system is shown. Modified from Boetius et al.<ref name= Boetius et al. 2015/>.]] | [[Image:Boeitus_et_al._2015_Diagram.png|thumb|300px|top|Fig. 2 Structure of subglacial environments, within the greater glacial system, is shown. Modified from Boetius et al.<ref name= "Boetius et al. 2015"/>.]] | ||
In addition to liquid water, the chemical components of subglacial minerals are required for microbial life. Due to the complete lack of light mentioned above, microbial communities rely on the presence of chemical energy within minerals at the ice-sediment interface. The flow of glaciers grinds these minerals, making them more available to the present microbial communities<ref name = Tranter et al. 2005>[https://onlinelibrary.wiley.com/doi/abs/10.1002/hyp.5854?casa_token=D8kWSvrpKYQAAAAA:0Selvba5GWf9VQEW3TUWiRtSqtaG1KegtOCslUpwHpEQeZkS-R5KNgEE1d3CCVJYNBaeeIw9HUqgE_Zr Tranter et al.: Hydrological control on microbial communities in subglacial environments. Hydrological Processes 2005, v. 19, p. 995-998.]</ref>. As a result, the minerology below glaciers and ice sheets is an important control on nutrient availability and thus community composition<ref name = Skidmore et al. 2005>[https://aem.asm.org/content/71/11/6986.short Skidmore et al.: Comparison of microbial community compositions of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Applied Environmental Microbiology 2005, v. 71, p. 6986-6997.]</ref>. | In addition to liquid water, the chemical components of subglacial minerals are required for microbial life. Due to the complete lack of light mentioned above, microbial communities rely on the presence of chemical energy within minerals at the ice-sediment interface. The flow of glaciers grinds these minerals, making them more available to the present microbial communities<ref name = Tranter et al. 2005>[https://onlinelibrary.wiley.com/doi/abs/10.1002/hyp.5854?casa_token=D8kWSvrpKYQAAAAA:0Selvba5GWf9VQEW3TUWiRtSqtaG1KegtOCslUpwHpEQeZkS-R5KNgEE1d3CCVJYNBaeeIw9HUqgE_Zr Tranter et al.: Hydrological control on microbial communities in subglacial environments. Hydrological Processes 2005, v. 19, p. 995-998.]</ref>. As a result, the minerology below glaciers and ice sheets is an important control on nutrient availability and thus community composition<ref name = "Skidmore et al. 2005">[https://aem.asm.org/content/71/11/6986.short Skidmore et al.: Comparison of microbial community compositions of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Applied Environmental Microbiology 2005, v. 71, p. 6986-6997.]</ref>. | ||
==Microbial Diversity== | |||
Despite the extreme conditions of subglacial environments, current research indicates the presence of diverse microbial communities<ref name = "Christner et al. 2014"> [https://www.nature.com/articles/nature13667 Christner et al.: A microbial ecosystem beneath the West Antarctic ice sheet. Nature 2014, v. 512, p. 310-313.]</ref><ref name= "Hamilton et al. 2013"/><ref name= "Boetius et al. 2015"/>. These communities can consist of bacteria, archaea, and eukarya<ref name= "Hamilton et al. 2013"/>. The diversity within and between subglacial environments is largely driven by bedrock and sediment mineralogy<ref name = "Mitchell et al. 2013"> [https://www.nature.com/articles/nature13667 Mitchell et al.: Influence of bedrock mineral composition on microbial diversity in a subglacial environment. Geology 2013, v. 41, no. 8, p. 855-858.]</ref><ref name= "Skidmore et al. 2005"/>, which drives chemolithoautotrophy within the system, the main source of primary productivity given the complete lack of sunlight<ref name= "Boetius et al. 2015"/>. | |||
This great microbial diversity of subglacial environments appears greater than other cryo-environments, such as supraglacial environments and snow (Hamilton et al. 2013; Boetius et al. 2015). Hamilton et al. (2013) suggested this greater level of diversity is driven by limited nutrient availability, requiring metabolic specificity, and resulting in “minimal niche overlap.” This minimal overlap allows for the proliferation of a variety of metabolically specific microbes. | |||
While data is limited (Boetius et al. 2015), the composition of subglacial communities appears to be characterized by relatively high bacterial abundance, specifically Betaproteobacteria, Deltaproteobacteria, Gammaproteobacteria, and Bacteroidetes (Hamilton et al. 2013; Boetius et al. 2015; Christner et al. 2014) (see fig x). Archaea appear present in most subglacial communities as well (Christner et al. 2014; Hamilton et al. 2013; Boetius et al. 2013), albeit with lower relative abundance than bacteria in at least some environments (Hamilton et al., 2013). Eukaryotes, while displaying high diversity when present (Hamilton et al., 2013), are not detectable in all subglacial environments (Christner et al. 2014). | |||
Since bedrock and sediment mineralogy drive chemolithoautotrophy and consequently microbial diversity within subglacial environments, the beta diversity of subglacial environments is partially reflective of mineralogical differences (Hamilton et al. 2013; Mitchell et al. 2013; Nixon et al., 2017; Skidmore et al. 2005) Much of this diversity appears to be driven specifically by the pyrite and Fe composition of bedrocks and sediments (Mitchell et al. 2013; Nixon et al., 2017). | |||
Revision as of 02:17, 12 June 2020
Detailed Environmental Description
Subglacial environments exist at the bed below ice sheets and glaciers. ~10% of land on Earth is covered by glacial ice[1] making subglacial environments a vast and important environment worthy of study. Glacial ice, while often associated with Earth’s poles, are also found well outside of polar regions[1] (Fig. 1), further signifying the expanse of subglacial environments on Earth.

The defining characteristics of subglacial environments include the complete lack of light[3], largely anoxic conditionsCite error: Invalid <ref> tag; invalid names, e.g. too manyCite error: Invalid <ref> tag; invalid names, e.g. too many, and low temperatures (around 0⁰C )[4]. Despite these common characteristics, subglacial environments are diverse in their environmental attributes. This partly derives from the diversity of Earth’s cryosphere. As suggested by the name, subglacial environments are found beneath glaciers, both alpine and outlet, but also below Earth’s massive ice sheets – the Antarctic and Greenlandic. These differing environments, while seemingly similar, are quite distinct and require their own fields of study. Glaciers only need be tens of meters thick, while ice sheets are kilometers thick.
Although defined by the presence of solid water (ice), many subglacial environments also contain liquid water – a required component for all life, including microbesCite error: Invalid <ref> tag; invalid names, e.g. too many. Below the ice of warm and polythermal glaciers high pressures result in liquid waterCite error: Invalid <ref> tag; invalid names, e.g. too many (see Fig 2.). The amount and distribution of this water can vary from saturated sediments, to localized channels, to subglacial lakesCite error: Invalid <ref> tag; invalid names, e.g. too many.
In addition to liquid water, the chemical components of subglacial minerals are required for microbial life. Due to the complete lack of light mentioned above, microbial communities rely on the presence of chemical energy within minerals at the ice-sediment interface. The flow of glaciers grinds these minerals, making them more available to the present microbial communitiesCite error: Invalid <ref> tag; invalid names, e.g. too many. As a result, the minerology below glaciers and ice sheets is an important control on nutrient availability and thus community composition[5].
Microbial Diversity
Despite the extreme conditions of subglacial environments, current research indicates the presence of diverse microbial communities[6][4][3]. These communities can consist of bacteria, archaea, and eukarya[4]. The diversity within and between subglacial environments is largely driven by bedrock and sediment mineralogy[7][5], which drives chemolithoautotrophy within the system, the main source of primary productivity given the complete lack of sunlight[3].
This great microbial diversity of subglacial environments appears greater than other cryo-environments, such as supraglacial environments and snow (Hamilton et al. 2013; Boetius et al. 2015). Hamilton et al. (2013) suggested this greater level of diversity is driven by limited nutrient availability, requiring metabolic specificity, and resulting in “minimal niche overlap.” This minimal overlap allows for the proliferation of a variety of metabolically specific microbes. While data is limited (Boetius et al. 2015), the composition of subglacial communities appears to be characterized by relatively high bacterial abundance, specifically Betaproteobacteria, Deltaproteobacteria, Gammaproteobacteria, and Bacteroidetes (Hamilton et al. 2013; Boetius et al. 2015; Christner et al. 2014) (see fig x). Archaea appear present in most subglacial communities as well (Christner et al. 2014; Hamilton et al. 2013; Boetius et al. 2013), albeit with lower relative abundance than bacteria in at least some environments (Hamilton et al., 2013). Eukaryotes, while displaying high diversity when present (Hamilton et al., 2013), are not detectable in all subglacial environments (Christner et al. 2014). Since bedrock and sediment mineralogy drive chemolithoautotrophy and consequently microbial diversity within subglacial environments, the beta diversity of subglacial environments is partially reflective of mineralogical differences (Hamilton et al. 2013; Mitchell et al. 2013; Nixon et al., 2017; Skidmore et al. 2005) Much of this diversity appears to be driven specifically by the pyrite and Fe composition of bedrocks and sediments (Mitchell et al. 2013; Nixon et al., 2017).
- ↑ 1.0 1.1 National Snow and Ice Data Center, 2020, Facts about glaciers. Accessed June 3, 2020.
- ↑ Randolph Glacier Inventory, 2017, GLIMS Viewer. Accessed June 3, 2020.
- ↑ 3.0 3.1 3.2 3.3 Boetius et al.: Microbial ecology of the cryosphere:sea ice and glacial habitats. Nature Reviews 2015, v. 13, p. 677-690.
- ↑ 4.0 4.1 4.2 Hamilton et al.: Molecular evidence for an active endogenous microbiome beneath glacial ice. The ISMEJ 2013, v.7, p. 1402-1412.
- ↑ 5.0 5.1 Skidmore et al.: Comparison of microbial community compositions of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Applied Environmental Microbiology 2005, v. 71, p. 6986-6997.
- ↑ Christner et al.: A microbial ecosystem beneath the West Antarctic ice sheet. Nature 2014, v. 512, p. 310-313.
- ↑ Mitchell et al.: Influence of bedrock mineral composition on microbial diversity in a subglacial environment. Geology 2013, v. 41, no. 8, p. 855-858.
