Microbiology of Hospital Washrooms: Difference between revisions
No edit summary |
No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
=Microorganisms in Washrooms= | =Microorganisms in Washrooms= | ||
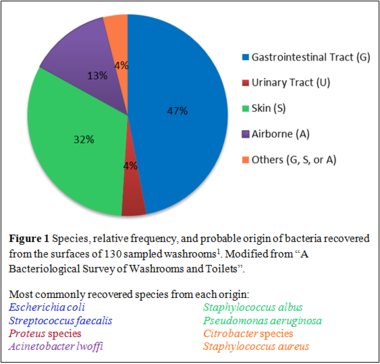
A bacteriological survey is commonly conducted following a disease outbreak to identify the etiology of the infection. Several studies have traced the source of infection to toilets [[#References|[1]]]; thus, understanding the microbiology of toilets is necessary to the prevention of bacterial infections. A study was conducted in which 130 washrooms from various premises were sampled to characterize the bacterial community (Figure 1) and infection “hotspots” (Figure 2). Human feces contain large populations of enteric facultative anaerobic bacteria such as ''Escherichia coli'' and '' | [[File:Washroom bacteria.png|380px|thumb|right|]] | ||
A bacteriological survey is commonly conducted following a disease outbreak to identify the etiology of the infection. Several studies have traced the source of infection to toilets [[#References|[1]]]; thus, understanding the microbiology of toilets is necessary to the prevention of bacterial infections. A study was conducted in which 130 washrooms from various premises were sampled to characterize the bacterial community (Figure 1) and infection “hotspots” (Figure 2). Human feces contain large populations of enteric facultative anaerobic bacteria such as ''[[Escherichia coli]]'' and ''[[Enterococcus faecalis]]''. On the other hand, obligate anaerobes and non-spore forming bacteria do not play a role in cross-infections in washrooms as they cannot survive on open surfaces [[#References|[1]]]. | |||
[[File:Washroom infection hotspots.png|380px|thumb|right|]] | |||
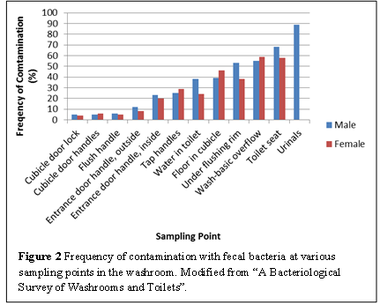
Urinals and toilet seats contained the greatest concentration of fecal bacteria (Figure 2), and are likely to be sources of infection. Surfaces that are normally dry, such as cubical door locks and flush handles, were rarely contaminated. On the other hand, inside handles of the entrance door and tap handles showed a greater degree of contamination since moisture from hands provides a more favourable environment for microorganisms [[#References|[1]]]. These surfaces are often touched by people after hand washing, which would re-contaminate their hands. | Urinals and toilet seats contained the greatest concentration of fecal bacteria (Figure 2), and are likely to be sources of infection. Surfaces that are normally dry, such as cubical door locks and flush handles, were rarely contaminated. On the other hand, inside handles of the entrance door and tap handles showed a greater degree of contamination since moisture from hands provides a more favourable environment for microorganisms [[#References|[1]]]. These surfaces are often touched by people after hand washing, which would re-contaminate their hands. | ||
=Disease Transmission= | =Disease Transmission= | ||
Methods of infection reported include splashing during defecation [[#References|[2]]], direct inhalation and ingestion [[#References|[3]]], as well as through the contaminated environment via the surface-to-hand-to-mouth contact [[#References|[4]]]. Microorganisms have been recovered in large enough numbers on various surfaces of the washroom such that all these methods of infection are possible [[#References|[1,2]]]. | Methods of infection reported include splashing during defecation [[#References|[2]]], direct inhalation and ingestion [[#References|[3]]], as well as through the contaminated environment via the surface-to-hand-to-mouth contact [[#References|[4]]]. Microorganisms have been recovered in large enough numbers on various surfaces of the washroom such that all these methods of infection are possible [[#References|[1,2]]]. | ||
| Line 21: | Line 18: | ||
==Prevalence of Microbes in Hospital Toilets== | ==Prevalence of Microbes in Hospital Toilets== | ||
The risk of infection arising from microorganisms breeding in hospital toilets has been evaluated; interestingly, opposing views exist on the likelihood and severity of such an infection. | The risk of infection arising from microorganisms breeding in hospital toilets has been evaluated; interestingly, opposing views exist on the likelihood and severity of such an infection. | ||
One of the first studies to evaluate the risk of infection from hospital toilets was conducted by Newsom in 1972. A low number of fecal bacteria were cultivated from the air, water, and surfaces of hospital toilets, indicating a low risk of infection. Free aerobic bacteria were too low in numbers, compared to the 10 | One of the first studies to evaluate the risk of infection from hospital toilets was conducted by Newsom in 1972. A low number of fecal bacteria were cultivated from the air, water, and surfaces of hospital toilets, indicating a low risk of infection. Free aerobic bacteria were too low in numbers, compared to the 10<sup>11</sup> required to produce a bacterial aerosol [[#References|[6]]], to be of concern. However, more recent studies have pointed out that Newsom did not account for either anaerobes or spore-forming bacteria. Newsom’s study predated the first case of ''[[Clostridium difficile]]'' infection, a species of spore-forming bacteria [[#References|[5]]]. Furthermore, Escherichia coli were used as the only indicator of fecal contamination [[#References|[1]]] when in reality there are many other types of enteric bacteria present. Therefore, the concentration of fecal bacteria in hospital toilets is likely to have been underestimated in Newsom’s study. | ||
=Antibiotic Resistant Pathogenic Species= | =Antibiotic Resistant Pathogenic Species= | ||
Because of the heavy use of antibiotics, many of the organisms found in hospital environments are resistant to antibiotics [[#References|[7]]]. Bacteria like methicillin-resistant ''Staphylococcus aureus'' (MRSA), vancomycin-resistant ''Enterococcus'' (VRE), and ''Clostridium difficile'' are becoming more prevalent in hospitals worldwide and pose a severe threat to immunocompromised patients and children [[#References|[8]]]. | Because of the heavy use of antibiotics, many of the organisms found in hospital environments are resistant to antibiotics [[#References|[7]]]. Bacteria like [http://en.wikipedia.org/wiki/Methicillin-resistant_Staphylococcus_aureus methicillin-resistant ''Staphylococcus aureus''] (MRSA), [http://en.wikipedia.org/wiki/Vancomycin-resistant_enterococcus vancomycin-resistant ''Enterococcus''] (VRE), and ''Clostridium difficile'' are becoming more prevalent in hospitals worldwide and pose a severe threat to immunocompromised patients and children [[#References|[8]]]. | ||
==Methicillin-resistant ''Staphylococcus aureus''== | ==Methicillin-resistant ''Staphylococcus aureus''== | ||
| Line 36: | Line 31: | ||
A study has recovered MRSA from 63% of cultures sampled from the environment near patients with heavy gastrointestinal (GI) colonization with MRSA compared to 33% from patients without GI colonization of MRSA. Furthermore, it was found that routine cleaning of contaminated environmental surfaces does not always eliminate MRSA from surfaces frequently touched by patients [[#References|[9]]], e.g. the toilet. | A study has recovered MRSA from 63% of cultures sampled from the environment near patients with heavy gastrointestinal (GI) colonization with MRSA compared to 33% from patients without GI colonization of MRSA. Furthermore, it was found that routine cleaning of contaminated environmental surfaces does not always eliminate MRSA from surfaces frequently touched by patients [[#References|[9]]], e.g. the toilet. | ||
Another study was conducted at a children’s hospital in 2009 to determine whether sharing toilet facilities could be a source of fomite transmission. MRSA was recovered at low colony counts on 3.3% of the toilets that were not cleaned with alcohol wipes. This was the first case in which MRSA was found on public toilets in outpatient areas of a hospital [[#References|[10]]]. | Another study was conducted at a children’s hospital in 2009 to determine whether sharing toilet facilities could be a source of [http://en.wikipedia.org/wiki/Fomite fomite transmission]. MRSA was recovered at low colony counts on 3.3% of the toilets that were not cleaned with alcohol wipes. This was the first case in which MRSA was found on public toilets in outpatient areas of a hospital [[#References|[10]]]. | ||
==''Clostridium difficile''== | ==''Clostridium difficile''== | ||
''Clostridium difficile'', an anaerobic Gram-positive rod [[#References|[7]]], is the most important cause of antibiotic-associated [http://en.wikipedia.org/wiki/Colitis colitis] (AAC), a condition caused by the overgrowth and toxin production of antibiotic-resistant organisms in the intestines [[#References|[11]]]. In the environment where patients with colitis were treated, around 10% of the 1086 cultures obtained from inanimate objects were positive for ''C. difficile''. In the washroom, ''C. difficile'' was recovered in samples from the toilet (31% of cultures were positive), bathroom floor (16%), and sinks (5%)11. Thus, a major vehicle of spread for ''C. difficile'' is infected patients with diarrhea [[#References|[7]]]. | |||
''Clostridium difficile'', an anaerobic Gram-positive rod [[#References|[7]]], is the most important cause of antibiotic-associated colitis (AAC), a condition caused by the overgrowth and toxin production of antibiotic-resistant organisms in the intestines [[#References|[11]]]. In the environment where patients with colitis were treated, around 10% of the 1086 cultures obtained from inanimate objects were positive for ''C. difficile''. In the washroom, ''C. difficile'' was recovered in samples from the toilet (31% of cultures were positive), bathroom floor (16%), and sinks (5%)11. Thus, a major vehicle of spread for ''C. difficile'' is infected patients with diarrhea [[#References|[7]]]. | |||
A study showed that ''C. diffcile'' was recovered from settle plates placed on the floor, cistern, and toilet seat during 90 min after flushing. Large droplets were released from the toilet and contaminated the surrounding environment quickly [[#References|[5]]]. Other researchers conducted an experiment in which the floor of the hospital room was inoculated with ''C. difficile'' and they were recovered for as long as five months. ''Clostridium difficile'' produces heat- and acid-resistant spores that can be inhaled or ingested [[#References|[11]]]. Not only do the spores deposit quickly onto surrounding surfaces, but also remain viable for a long time. More is yet to be learned about the epidemiology and prevention of antibiotic-associated colitis in hospitals [[#References|[11]]]. | A study showed that ''C. diffcile'' was recovered from settle plates placed on the floor, cistern, and toilet seat during 90 min after flushing. Large droplets were released from the toilet and contaminated the surrounding environment quickly [[#References|[5]]]. Other researchers conducted an experiment in which the floor of the hospital room was inoculated with ''C. difficile'' and they were recovered for as long as five months. ''Clostridium difficile'' produces heat- and acid-resistant spores that can be inhaled or ingested [[#References|[11]]]. Not only do the spores deposit quickly onto surrounding surfaces, but also remain viable for a long time. More is yet to be learned about the epidemiology and prevention of antibiotic-associated colitis in hospitals [[#References|[11]]]. | ||
| Line 50: | Line 43: | ||
==Prevention== | ==Prevention== | ||
Some researchers suggest that cleaning of toilets once a day is sufficient to prevent the spread of disease provided that the toilets are cleaned immediately when obviously soiled [[#References|[12]]]. However, more recent studies have shown that routine cleaning of contaminated environmental surfaces does not always eliminate pathogenic bacteria (e.g. MRSA) from surfaces frequently touched by patients. It has also been shown that enhanced cleaning methods, such as using hydrogen peroxide vapour, has resulted in a decrease of ''C. difficile'' contamination [[#References|[9]]]. Furthermore, the lidless toilets commonly seen in hospitals increase the risk of ''C. difficile'' contamination [[#References|[5]]]. | [[File:Lidless toilets.jpeg|thumb|380px|right|Lidless toilets]] | ||
Some researchers suggest that cleaning of toilets once a day is sufficient to prevent the spread of disease provided that the toilets are cleaned immediately when obviously soiled [[#References|[12]]]. However, more recent studies have shown that routine cleaning of contaminated environmental surfaces does not always eliminate pathogenic bacteria (e.g. MRSA) from surfaces frequently touched by patients. It has also been shown that enhanced cleaning methods, such as using [http://en.wikipedia.org/wiki/Hydrogen_peroxide hydrogen peroxide vapour], has resulted in a decrease of ''C. difficile'' contamination [[#References|[9]]]. Furthermore, the lidless toilets commonly seen in hospitals increase the risk of ''C. difficile'' contamination [[#References|[5]]]. | |||
==Handwashing== | ==Handwashing== | ||
Besides maintaining the hospitals washroom facilities clean, appropriate hand hygiene is paramount in preventing nosocomial infections [[#References|[6]]]. Inadequate hand hygiene is the leading cause of hospital-associated infections and spread of antibiotic-resistant microorganisms [[#References|[13]]]. However, the hand hygiene compliance among healthcare workers (only males were observed) is below 50%, lower than users of public washrooms [[#References|[13]]]. In another study, it was shown that only 30% of physicians adhered to hand-hygiene practices, compared to 48% of the average healthcare worker. Furthermore, the proportion of compliance was lower in intensive care units, during procedures with a high risk of contamination, and when more patients needed treatment [[#References|[14]]]. It is speculated that the low compliance rate is due to understaffing [[#References|[14]]], and that the relatively clean hospital environment gives a false impression of safety to healthcare workers [[#References|[13]]]. | [[File:Handwashing.jpeg|thumb|380px|right|Hand hygiene]] | ||
Besides maintaining the hospitals washroom facilities clean, appropriate hand hygiene is paramount in preventing [http://en.wikipedia.org/wiki/Nosocomial_infections nosocomial infections] [[#References|[6]]]. Inadequate hand hygiene is the leading cause of hospital-associated infections and spread of antibiotic-resistant microorganisms [[#References|[13]]]. However, the hand hygiene compliance among healthcare workers (only males were observed) is below 50%, lower than users of public washrooms [[#References|[13]]]. In another study, it was shown that only 30% of physicians adhered to hand-hygiene practices, compared to 48% of the average healthcare worker. Furthermore, the proportion of compliance was lower in intensive care units, during procedures with a high risk of contamination, and when more patients needed treatment [[#References|[14]]]. It is speculated that the low compliance rate is due to understaffing [[#References|[14]]], and that the relatively clean hospital environment gives a false impression of safety to healthcare workers [[#References|[13]]]. | |||
=References= | =References= | ||
Latest revision as of 09:46, 12 December 2012
Microorganisms in Washrooms
A bacteriological survey is commonly conducted following a disease outbreak to identify the etiology of the infection. Several studies have traced the source of infection to toilets [1]; thus, understanding the microbiology of toilets is necessary to the prevention of bacterial infections. A study was conducted in which 130 washrooms from various premises were sampled to characterize the bacterial community (Figure 1) and infection “hotspots” (Figure 2). Human feces contain large populations of enteric facultative anaerobic bacteria such as Escherichia coli and Enterococcus faecalis. On the other hand, obligate anaerobes and non-spore forming bacteria do not play a role in cross-infections in washrooms as they cannot survive on open surfaces [1].
Urinals and toilet seats contained the greatest concentration of fecal bacteria (Figure 2), and are likely to be sources of infection. Surfaces that are normally dry, such as cubical door locks and flush handles, were rarely contaminated. On the other hand, inside handles of the entrance door and tap handles showed a greater degree of contamination since moisture from hands provides a more favourable environment for microorganisms [1]. These surfaces are often touched by people after hand washing, which would re-contaminate their hands.
Disease Transmission
Methods of infection reported include splashing during defecation [2], direct inhalation and ingestion [3], as well as through the contaminated environment via the surface-to-hand-to-mouth contact [4]. Microorganisms have been recovered in large enough numbers on various surfaces of the washroom such that all these methods of infection are possible [1,2].
The release of microorganisms into the air is most likely immediately after first flushing, i.e. the concentration of bacteria recovered from air samples is greatest. Even though each subsequent flush allowed a 2 to 3-log reduction in the concentration of microbes, it caused a further dissemination of airborne particles [4]. Surface contamination occurred within 90 min of flushing [5].
Clinical Relevance
Prevalence of Microbes in Hospital Toilets
The risk of infection arising from microorganisms breeding in hospital toilets has been evaluated; interestingly, opposing views exist on the likelihood and severity of such an infection.
One of the first studies to evaluate the risk of infection from hospital toilets was conducted by Newsom in 1972. A low number of fecal bacteria were cultivated from the air, water, and surfaces of hospital toilets, indicating a low risk of infection. Free aerobic bacteria were too low in numbers, compared to the 1011 required to produce a bacterial aerosol [6], to be of concern. However, more recent studies have pointed out that Newsom did not account for either anaerobes or spore-forming bacteria. Newsom’s study predated the first case of Clostridium difficile infection, a species of spore-forming bacteria [5]. Furthermore, Escherichia coli were used as the only indicator of fecal contamination [1] when in reality there are many other types of enteric bacteria present. Therefore, the concentration of fecal bacteria in hospital toilets is likely to have been underestimated in Newsom’s study.
Antibiotic Resistant Pathogenic Species
Because of the heavy use of antibiotics, many of the organisms found in hospital environments are resistant to antibiotics [7]. Bacteria like methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and Clostridium difficile are becoming more prevalent in hospitals worldwide and pose a severe threat to immunocompromised patients and children [8].
Methicillin-resistant Staphylococcus aureus
A study has recovered MRSA from 63% of cultures sampled from the environment near patients with heavy gastrointestinal (GI) colonization with MRSA compared to 33% from patients without GI colonization of MRSA. Furthermore, it was found that routine cleaning of contaminated environmental surfaces does not always eliminate MRSA from surfaces frequently touched by patients [9], e.g. the toilet.
Another study was conducted at a children’s hospital in 2009 to determine whether sharing toilet facilities could be a source of fomite transmission. MRSA was recovered at low colony counts on 3.3% of the toilets that were not cleaned with alcohol wipes. This was the first case in which MRSA was found on public toilets in outpatient areas of a hospital [10].
Clostridium difficile
Clostridium difficile, an anaerobic Gram-positive rod [7], is the most important cause of antibiotic-associated colitis (AAC), a condition caused by the overgrowth and toxin production of antibiotic-resistant organisms in the intestines [11]. In the environment where patients with colitis were treated, around 10% of the 1086 cultures obtained from inanimate objects were positive for C. difficile. In the washroom, C. difficile was recovered in samples from the toilet (31% of cultures were positive), bathroom floor (16%), and sinks (5%)11. Thus, a major vehicle of spread for C. difficile is infected patients with diarrhea [7].
A study showed that C. diffcile was recovered from settle plates placed on the floor, cistern, and toilet seat during 90 min after flushing. Large droplets were released from the toilet and contaminated the surrounding environment quickly [5]. Other researchers conducted an experiment in which the floor of the hospital room was inoculated with C. difficile and they were recovered for as long as five months. Clostridium difficile produces heat- and acid-resistant spores that can be inhaled or ingested [11]. Not only do the spores deposit quickly onto surrounding surfaces, but also remain viable for a long time. More is yet to be learned about the epidemiology and prevention of antibiotic-associated colitis in hospitals [11].
Hygiene
Prevention
Some researchers suggest that cleaning of toilets once a day is sufficient to prevent the spread of disease provided that the toilets are cleaned immediately when obviously soiled [12]. However, more recent studies have shown that routine cleaning of contaminated environmental surfaces does not always eliminate pathogenic bacteria (e.g. MRSA) from surfaces frequently touched by patients. It has also been shown that enhanced cleaning methods, such as using hydrogen peroxide vapour, has resulted in a decrease of C. difficile contamination [9]. Furthermore, the lidless toilets commonly seen in hospitals increase the risk of C. difficile contamination [5].
Handwashing
Besides maintaining the hospitals washroom facilities clean, appropriate hand hygiene is paramount in preventing nosocomial infections [6]. Inadequate hand hygiene is the leading cause of hospital-associated infections and spread of antibiotic-resistant microorganisms [13]. However, the hand hygiene compliance among healthcare workers (only males were observed) is below 50%, lower than users of public washrooms [13]. In another study, it was shown that only 30% of physicians adhered to hand-hygiene practices, compared to 48% of the average healthcare worker. Furthermore, the proportion of compliance was lower in intensive care units, during procedures with a high risk of contamination, and when more patients needed treatment [14]. It is speculated that the low compliance rate is due to understaffing [14], and that the relatively clean hospital environment gives a false impression of safety to healthcare workers [13].
References
[1] Mendes, M.P. and Lynch, D.J. “A bacteriological survey of washrooms and toilets.” Journal of Hygiene, 1976, DOI:10.1017/S002217240005508X
[2] Burgess, J.A. "Trichomonas vaginalis infection from splashing in water closets." The British Journal of Venereal Diseases, 1963, DOI:10.1136/sti.39.4.248
[3] Darlow, H.M. and Bale, W.R. "Infective hazards of water-closets." Lancet, 1959, DOI: 10.1016/S0140-6736(59)91201-2
[4] Barker, J. and Jones, M. V. “The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet.” Journal of Applied Microbiology, 2005, DOI: 10.1111/j.1365-2672.2005.02610.x
[5] Best, E.L., Sandoe, J.A.T. and Wilcox, M.H. “Potential for aerosolization of Clostridium difficile after flushing toilets: the role of toilet lids in reducing environmental contamination risk.” Journal of Hospital Infection, 2011, DOI: 10.1016/j.jhin.2011.08.010
[6] Newsom, S.W.B. "Microbiology of hospital toilets." The Lancet, 1972, DOI: 10.1016/S0140-6736(72)92102-2
[7] Dancer, S.J. "Mopping up hospital infection." Journal of hospital infection, 1999, DOI: 0195–6701/99/100085
[8] Johnston, B.L. and Bryce, E. “Hospital infection control strategies for vancomycin-resistant Enterococcus, methicillin-resistant Staphylococcus aureus and Clostridium difficile. Canadian Medical Association Journal, 2009, DOI: 10.1503/cmaj.080195
[9] Boyce, J.M. “Environmental contamination makes an important contribution to hospital infection.” Journal of Hospital Infection, 2007, DOI: 0195-6701/$
[10] Giannini, M. A., Nance, D. and McCullers, J. A. "Are toilet seats a vector for transmission of methicillin-resistant Staphylococcus aureus?" American Journal of Infection Control, 2009, DOI: 10.1016/j.ajic.2008.11.005
[11] Fekety, R., Kim, K.H., Brown, D., Batts, D.H., Cudmore, M. and Silva, J. “Epidemiology of antibiotic-associated colitis: isolation of Clostridium difficile from the hospital environment.” The American journal of medicine, 1981, DOI: 10.1016/0002-9343(81)90553-2
[12] Hambraeus, A. and Malmborg, A.S. "Disinfection or cleaning of hospital toilets—an evaluation of different routines." Journal of Hospital Infection, 1980, DOI: 10.1016/0195-6701(80)90048-1
[13] Van der Vegt, D.S.J.M. and Voss, A. "Are hospitals too clean to trigger good hand hygiene?" Journal of Hospital Infection, 2009, DOI: 10.1016/j.jhin.2009.03.006
[14] Pittet, D., Mourouga, P. and Perneger, T.V. "Compliance with handwashing in a teaching hospital." Annals of internal medicine, 1999.