Microvirgula aerodenitrificans: Difference between revisions
No edit summary |
|||
| (49 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{curated}} | ||
==Classification== | ==Classification== | ||
| Line 9: | Line 5: | ||
Domain: Bacteria | Domain: Bacteria | ||
Phylum: Proteobacteria | Phylum: ''Proteobacteria'' | ||
Class: Betaproteobacteria | Class: ''[http://en.wikipedia.org/wiki/Betaproteobacteria Betaproteobacteria]'' | ||
Order: Neisseriales | Order: ''Neisseriales'' | ||
Family: Neisseriaceae | Family: ''Neisseriaceae'' | ||
Genus: Microvirgula | Genus: ''Microvirgula'' | ||
Species: aerodenitrificans | Species: ''aerodenitrificans'' | ||
== | ===Species=== | ||
''Microvirgula aerodenitrificans'' | |||
==Introduction== | |||
''M. aerodenitrificans'' are abile to performs [http://en.wikipedia.org/wiki/Denitrification denitrification] under [http://en.wikipedia.org/wiki/Aerobic aerobic] condition [1,3,5]. While most denitrifiers utilize nitrate and nitrite as terminal electron acceptors under [http://en.wikipedia.org/wiki/Anaerobic anaerobic] conditions, , ''M. aerodenitrificans'' reduces both oxygen and nitrogen simultaneously when oxygen is present [5]. However, the biological significance is not defined yet for aerobic denitrification. The habitat for this organism is not specified and found in a various places globally where selective pressure such as fluctuating oxygen concentration occurs[7,8]. These places are mostly sludge, mixture of wastewater treatment and also found in canal and a pond [8]. | |||
Until 2012, ''Microvirgula aerodenitrificans'' has not been described as a causative organism of clinical infection. However, the first human case has been recently reported in where ''M. aerodenitrificans'' was linked to [http://en.wikipedia.org/wiki/Bacteremia bacteremia] in a 15-month-old infant boy with Pompe’s disease. The disease causality of the organism itself is not yet proven but it is shown that such organism can influence vascular access mechanism and may lead to a clinical disease especially for a person with an [http://en.wikipedia.org/wiki/Immunodeficiency immunodeficiency] [2]. | |||
== | ==Morphology and Classification== | ||
[[File:Bipolar Flagellation.jpeg|400px|thumb|right|Bipolar Flagellation]] | |||
''Microvirgula aerodenitrificans'' was first isolated from an activated sludge in 1998 by Patureau et al. This microorganism is a curved, rod shaped ([http://en.wikipedia.org/wiki/Vibrio vibrio]), motile, [http://en.wikipedia.org/wiki/Gram-negative_bacteria gram negative], [http://en.wikipedia.org/wiki/Catalase catalase]- and [http://en.wikipedia.org/wiki/Oxidase oxidase]- positive, and aerobically denitrifying bacteria [5]. It is also a [http://en.wikipedia.org/wiki/Mesophile mesophile] and [http://goldbook.iupac.org/N04133.html neutrophilic organism], where the maximal growth occurs at 35oC and pH 7 [5]. The organism still shows growth at temperature range between 15oC to 45oC and under pH 6 [5]. The size of the cell varies within a [http://en.wikipedia.org/wiki/Bacterial_growth growth stage] [5]. At the beginning of the growth stage, it has a thin shape but becomes larger and associate with 4-5 cells by the late [http://en.wikipedia.org/wiki/Stationary_phase_(biology) stationary phase] [5]. Based on these [http://en.wikipedia.org/wiki/Phenotype phenotypic] characteristics, ''M. aerodenitrificans'' is the most close to ''Cornarnonas testosteroni'' and ''[http://en.wikipedia.org/wiki/Pseudomonas Pseudomonas] alcaligenes'' [1, 5]. | |||
''M. aerodenitrificans'' is a typical Gram negative cell wall structure, which includes two layers of membranes, outer and inner, revealed with an undulating outer membrane [5]. The isolated colonies after a 24 h [http://en.wikipedia.org/wiki/Incubation_period incubation period] were shown to be circular with 1±2 mm diameter and cream-coloured [5] In young cultures, cells occurred as singly or in pairs. Under a [http://en.wikipedia.org/wiki/Negative_stain negative staining] [http://en.wikipedia.org/wiki/Electron_microscope electron microscopy], a thin section of ''M. aerodenitrificans'' possesses bipolar tufts of [http://en.wikipedia.org/wiki/Flagellum flagella] [5]. | |||
==Metabolism== | |||
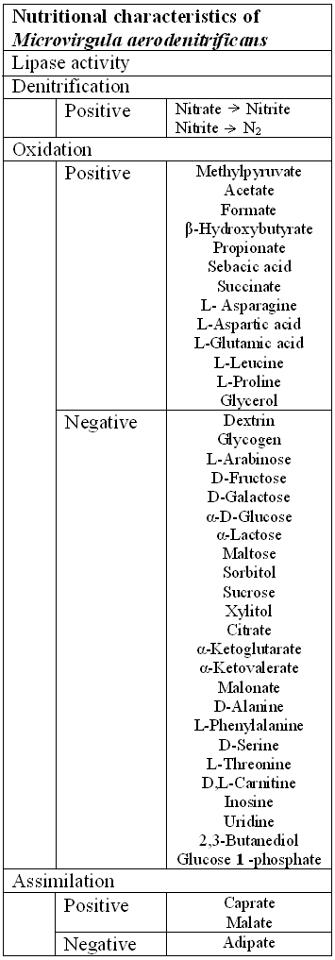
[[File:Nutritional characteristics table.jpeg|400px|thumb|left|Nutritional characteristics of ''Microvirgula aerodenitrificans'']] | |||
''Microvirgula aerodenitrificans'' is a Gram-negative and slightly curved rod shaped bacteria where ''Microvirgula aerodenitrificans'' is an aerobic and [http://en.wikipedia.org/wiki/Heterotroph heterotrophic] denitrifier [7]. The organism obtains both carbon and electron sources from different kinds of organic matters; however, it has been shown that this organism can’t readily oxidize sugar compounds [1]. In addition, ''M. aerodenitrificans'' is shown to be able to digest and use caprate, malate and hydrolyse arginine based on the API 20 NE tests [1]. In its usual habitat, sludge or a waste treatment, acetate would be used as a common carbon/electron source for [http://en.wikipedia.org/wiki/Metabolism metabolism] [7]. In addition, as a denitrifier, the organism uses oxidized forms of nitrogen as terminal [http://en.wikipedia.org/wiki/Electron_acceptor electron acceptor] (TEA) and produces gaseous nitrogen oxide products [10]. Furthermore, unlike other denitrifiers, ''M. aerodenitrificans'' performs an aerobic denitrification which is a co-[http://en.wikipedia.org/wiki/Cellular_respiration respiration] of the two electron acceptors, oxygen and nitrogen compound, at the same time [5]. | |||
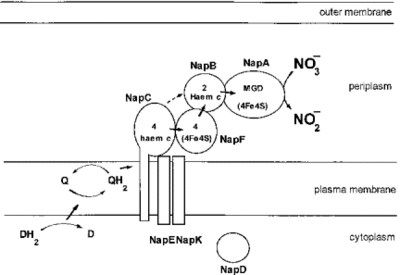
[[File:Periplasmic nitrate reductase.gif|400px|thumb|right|Periplasmic nitrate reductase]] | |||
== | ==Aerobic denitrification== | ||
[[ | Denitrification is generally understood to take place under anaerobic condition since oxygen is theoretically more energy yielding [http://en.wikipedia.org/wiki/Oxidizing_agent oxidizing agent] and inhibits the denitrification activity [3,9]. However, aerobic denitrifying organisms are indeed found in a broad range of microbial organisms with different levels of productivity. [9,10] ''M. aerodenitrificans'' is also an aerobic denitrifier which is capable of using two electron acceptors, oxygen and nitrogen, simultaneously. In the presence of oxygen, ''M. aerodenitrificans'' actively use oxygen as a terminal electron acceptor (TEA) and also consume a considerable amount of oxidized nitrogen compounds at significant reduction rates [6]. The end products are either Nitrous oxide (N2O) or Dinitrogen (N2) [1,9] and this process is accounted by a [http://en.wikipedia.org/wiki/Periplasmic_space periplasmic] [http://en.wikipedia.org/wiki/Nitrate_reductase nitrate reductase] in ''M. aerodenitrificans'' [3,4]. Even though nitrate reduction is observed in aerobic denitrifiers, the ability of denitrifying activity is dependent on few factors such as concentration of carbon sources nearby, oxygen and bacterial density [3,7]. According to Y. Otani ''et al.'' sufficient amount of carbon concentration or high enough concentration of bacterial density is required so that the aerobic denitrifiers can show the nitrate reduction activity under aerobic conditions [3,7]. Furthermore, decreased amount of oxygen boost the aerobic denitrification [7]. The significant biological advantages are not yet well proven; however, aerobic denitrifiers have an advantage over anaerobic denitrifiers by having a greater adaptability to the environment with fluctuating oxygen level. | ||
[[File:N cycle.jpeg|400px|thumb|Bottom|Nitrogen cycle]] | |||
==Ecology== | ==Ecology== | ||
Denitrification is a critical step of the [http://en.wikipedia.org/wiki/Nitrogen_cycle nitrogen cycle] in which bacteria recycle oxidized nitrogen compounds to dinitrogen. Denitrification itself contributes to many critical environmental consequences such as loss of fixed nitrogen from [http://en.wikipedia.org/wiki/Biosphere biosphere], agricultural soil and cause of the [http://en.wikipedia.org/wiki/Greenhouse_effect greenhouse effect] etc. Aerobic denitrifying organisms specifically affect the [http://en.wikipedia.org/wiki/Nitrification nitrification] rate in the mixed culture with nitrifying organisms that the higher quantities of aerobic denitrifiers cause reduced nitrifying activity. [4] | |||
== | ==Potential Application== | ||
[[File:sludge treatment.jpeg|400px|thumb|Right|Aerobic Sludge Treatment]] | |||
In a process for sewage and industrial [http://en.wikipedia.org/wiki/Sewage_treatment wastewater treatment], where simultaneous removal of carbon and nitrogen is required, aerobic denitrifiers can be implied by making the process more efficient. The conventional wastewater treatment consists of two steps, aerobic nitrification by autotrophs and denitrification under anaerobic condition, making the system more costly and complex [10]. However, the ability of Microvirgula aerodenitrificans to aerobically denitrify can be applied by achieving a nitrogen reduction without further system modification from aerobic condition to anaerobic condition. [7] | |||
==References== | |||
[1] Cleenwerck I., De Wachter M., Hoste B., Janssens D., and Swings J. “Aquaspirillum dispar Hylemon et al. 1973 and Microvirgula aerodenitrificans Patureau et al. 1998 are subjective synonyms” IJSEM, 2003, 53:1457-1459. | |||
== | |||
[2] Murphy M. E., Goodson A., Malnick H., Shah J., Neelamkavil R., and Devi R. “Recurrent Microvirgula aerodenitrificans Bacteremia” J. Clin. Microbiol, 2012, 50(8):2823. DOI: 10.1128/JCM.00392-12. | |||
[ | [3] Otani Y., Hasegawa K., and Hanaki K. “Comparison of Aerobic Denitrifying Activity Among Three Cultural Species With Various Carbon Sources” Water Science and Technology, 2004, 50:15-22. | ||
[ | [4] Patureau D., Bernet N., Bouchez T., Dabert P., Delgenes J.P., and Moletta R. “Biological Nitrogen Removal In a Single Aerobic Reactor By Association of a Nitrifying Ecosystem To an Aerobic Denitrifier, Microvirgula aerodenitrificans” Journal of Molecular Catalysis B: Enzymatic, 1998, 435-439. | ||
[ | [5] Patureau D., Godon JJ, Dabert P, Bouchez T, Bernet N, Delgenes JP. “Microvirgula aerodenitrificans gen. nov., sp. Nov., a New Gram-negative Bacterium Exhibiting Co-respiration of Oxygen and Nitrogen oxides up to Oxygen-saturated Conditions” International Journal of Systematic Bacteriology, 1998, 48. 775-782. | ||
[ | [6] Patureau D., Bernet N., Delgenes JP., and Moletta R. “Effect of Dissolved Oxygen And Carbon-Nitrogen Loads On Denitrifaction By Aerobic Consortium” Appl. Microbiol Biotechnol 2000, 54(4): 535-542. | ||
[ | [7] Patureau D., Helloin E., Rustrian E., Bouchez T. Delgenes J.P., and Moletta R. “Combined Phosphate and Nitrogen Removal in a Sequencing Batch Reactor Using The Aerobic Denitrifier, Microvirgula aerodenitrificans” Wat. Res., 2001, 35(1): 189–197. | ||
[ | [8] Patureau D., Zumstein E., Delgenes J. P., and Moletta R. “Aerobic Denitrifiers Isolated from Diverse Natural and Managed Ecosystems” Microbial Ecology. 2000, 39(2): 145-152. | ||
[ | [9] Lloyd D. “Aerobic Denitrification in Soils and Sediments: From Fallacies to Facts” Trends in Ecology & Evolution, 1993, 8(10): 352-356. | ||
[ | [10] Zhu L., Ding W., Feng L.J., Kong Y., Xu J., and Xu X.Y. “Isolation of Aerobic Denitrifiers And Characterization For Their Potential Application In The Bioremediation of Oligotrophic Ecosystem” Bioresource Technology, 2012, 108: 1-7. | ||
==Author== | |||
Page authored by Jia suh, student of University of British Columbia | |||
Latest revision as of 05:15, 27 December 2012
Classification
Domain: Bacteria
Phylum: Proteobacteria
Class: Betaproteobacteria
Order: Neisseriales
Family: Neisseriaceae
Genus: Microvirgula
Species: aerodenitrificans
Species
Microvirgula aerodenitrificans
Introduction
M. aerodenitrificans are abile to performs denitrification under aerobic condition [1,3,5]. While most denitrifiers utilize nitrate and nitrite as terminal electron acceptors under anaerobic conditions, , M. aerodenitrificans reduces both oxygen and nitrogen simultaneously when oxygen is present [5]. However, the biological significance is not defined yet for aerobic denitrification. The habitat for this organism is not specified and found in a various places globally where selective pressure such as fluctuating oxygen concentration occurs[7,8]. These places are mostly sludge, mixture of wastewater treatment and also found in canal and a pond [8]. Until 2012, Microvirgula aerodenitrificans has not been described as a causative organism of clinical infection. However, the first human case has been recently reported in where M. aerodenitrificans was linked to bacteremia in a 15-month-old infant boy with Pompe’s disease. The disease causality of the organism itself is not yet proven but it is shown that such organism can influence vascular access mechanism and may lead to a clinical disease especially for a person with an immunodeficiency [2].
Morphology and Classification
Microvirgula aerodenitrificans was first isolated from an activated sludge in 1998 by Patureau et al. This microorganism is a curved, rod shaped (vibrio), motile, gram negative, catalase- and oxidase- positive, and aerobically denitrifying bacteria [5]. It is also a mesophile and neutrophilic organism, where the maximal growth occurs at 35oC and pH 7 [5]. The organism still shows growth at temperature range between 15oC to 45oC and under pH 6 [5]. The size of the cell varies within a growth stage [5]. At the beginning of the growth stage, it has a thin shape but becomes larger and associate with 4-5 cells by the late stationary phase [5]. Based on these phenotypic characteristics, M. aerodenitrificans is the most close to Cornarnonas testosteroni and Pseudomonas alcaligenes [1, 5]. M. aerodenitrificans is a typical Gram negative cell wall structure, which includes two layers of membranes, outer and inner, revealed with an undulating outer membrane [5]. The isolated colonies after a 24 h incubation period were shown to be circular with 1±2 mm diameter and cream-coloured [5] In young cultures, cells occurred as singly or in pairs. Under a negative staining electron microscopy, a thin section of M. aerodenitrificans possesses bipolar tufts of flagella [5].
Metabolism
Microvirgula aerodenitrificans is a Gram-negative and slightly curved rod shaped bacteria where Microvirgula aerodenitrificans is an aerobic and heterotrophic denitrifier [7]. The organism obtains both carbon and electron sources from different kinds of organic matters; however, it has been shown that this organism can’t readily oxidize sugar compounds [1]. In addition, M. aerodenitrificans is shown to be able to digest and use caprate, malate and hydrolyse arginine based on the API 20 NE tests [1]. In its usual habitat, sludge or a waste treatment, acetate would be used as a common carbon/electron source for metabolism [7]. In addition, as a denitrifier, the organism uses oxidized forms of nitrogen as terminal electron acceptor (TEA) and produces gaseous nitrogen oxide products [10]. Furthermore, unlike other denitrifiers, M. aerodenitrificans performs an aerobic denitrification which is a co-respiration of the two electron acceptors, oxygen and nitrogen compound, at the same time [5].
Aerobic denitrification
Denitrification is generally understood to take place under anaerobic condition since oxygen is theoretically more energy yielding oxidizing agent and inhibits the denitrification activity [3,9]. However, aerobic denitrifying organisms are indeed found in a broad range of microbial organisms with different levels of productivity. [9,10] M. aerodenitrificans is also an aerobic denitrifier which is capable of using two electron acceptors, oxygen and nitrogen, simultaneously. In the presence of oxygen, M. aerodenitrificans actively use oxygen as a terminal electron acceptor (TEA) and also consume a considerable amount of oxidized nitrogen compounds at significant reduction rates [6]. The end products are either Nitrous oxide (N2O) or Dinitrogen (N2) [1,9] and this process is accounted by a periplasmic nitrate reductase in M. aerodenitrificans [3,4]. Even though nitrate reduction is observed in aerobic denitrifiers, the ability of denitrifying activity is dependent on few factors such as concentration of carbon sources nearby, oxygen and bacterial density [3,7]. According to Y. Otani et al. sufficient amount of carbon concentration or high enough concentration of bacterial density is required so that the aerobic denitrifiers can show the nitrate reduction activity under aerobic conditions [3,7]. Furthermore, decreased amount of oxygen boost the aerobic denitrification [7]. The significant biological advantages are not yet well proven; however, aerobic denitrifiers have an advantage over anaerobic denitrifiers by having a greater adaptability to the environment with fluctuating oxygen level.
Ecology
Denitrification is a critical step of the nitrogen cycle in which bacteria recycle oxidized nitrogen compounds to dinitrogen. Denitrification itself contributes to many critical environmental consequences such as loss of fixed nitrogen from biosphere, agricultural soil and cause of the greenhouse effect etc. Aerobic denitrifying organisms specifically affect the nitrification rate in the mixed culture with nitrifying organisms that the higher quantities of aerobic denitrifiers cause reduced nitrifying activity. [4]
Potential Application
In a process for sewage and industrial wastewater treatment, where simultaneous removal of carbon and nitrogen is required, aerobic denitrifiers can be implied by making the process more efficient. The conventional wastewater treatment consists of two steps, aerobic nitrification by autotrophs and denitrification under anaerobic condition, making the system more costly and complex [10]. However, the ability of Microvirgula aerodenitrificans to aerobically denitrify can be applied by achieving a nitrogen reduction without further system modification from aerobic condition to anaerobic condition. [7]
References
[1] Cleenwerck I., De Wachter M., Hoste B., Janssens D., and Swings J. “Aquaspirillum dispar Hylemon et al. 1973 and Microvirgula aerodenitrificans Patureau et al. 1998 are subjective synonyms” IJSEM, 2003, 53:1457-1459.
[2] Murphy M. E., Goodson A., Malnick H., Shah J., Neelamkavil R., and Devi R. “Recurrent Microvirgula aerodenitrificans Bacteremia” J. Clin. Microbiol, 2012, 50(8):2823. DOI: 10.1128/JCM.00392-12.
[3] Otani Y., Hasegawa K., and Hanaki K. “Comparison of Aerobic Denitrifying Activity Among Three Cultural Species With Various Carbon Sources” Water Science and Technology, 2004, 50:15-22.
[4] Patureau D., Bernet N., Bouchez T., Dabert P., Delgenes J.P., and Moletta R. “Biological Nitrogen Removal In a Single Aerobic Reactor By Association of a Nitrifying Ecosystem To an Aerobic Denitrifier, Microvirgula aerodenitrificans” Journal of Molecular Catalysis B: Enzymatic, 1998, 435-439.
[5] Patureau D., Godon JJ, Dabert P, Bouchez T, Bernet N, Delgenes JP. “Microvirgula aerodenitrificans gen. nov., sp. Nov., a New Gram-negative Bacterium Exhibiting Co-respiration of Oxygen and Nitrogen oxides up to Oxygen-saturated Conditions” International Journal of Systematic Bacteriology, 1998, 48. 775-782.
[6] Patureau D., Bernet N., Delgenes JP., and Moletta R. “Effect of Dissolved Oxygen And Carbon-Nitrogen Loads On Denitrifaction By Aerobic Consortium” Appl. Microbiol Biotechnol 2000, 54(4): 535-542.
[7] Patureau D., Helloin E., Rustrian E., Bouchez T. Delgenes J.P., and Moletta R. “Combined Phosphate and Nitrogen Removal in a Sequencing Batch Reactor Using The Aerobic Denitrifier, Microvirgula aerodenitrificans” Wat. Res., 2001, 35(1): 189–197.
[8] Patureau D., Zumstein E., Delgenes J. P., and Moletta R. “Aerobic Denitrifiers Isolated from Diverse Natural and Managed Ecosystems” Microbial Ecology. 2000, 39(2): 145-152.
[9] Lloyd D. “Aerobic Denitrification in Soils and Sediments: From Fallacies to Facts” Trends in Ecology & Evolution, 1993, 8(10): 352-356.
[10] Zhu L., Ding W., Feng L.J., Kong Y., Xu J., and Xu X.Y. “Isolation of Aerobic Denitrifiers And Characterization For Their Potential Application In The Bioremediation of Oligotrophic Ecosystem” Bioresource Technology, 2012, 108: 1-7.
Author
Page authored by Jia suh, student of University of British Columbia