Mount Erebus
Introduction
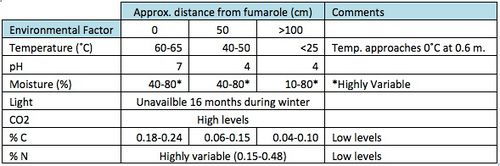
Mount Erebus is a volcano located in Ross Island, Antarctica, with an elevation of 3,794 m. The area near the summit is home to several extreme environments. Microbial growth in these environments is constrained by wide temperature ranges, reduced water activity [1] and pH [2]. Yet, studies on geothermal soils on Mt. Erebus have found that bacteria are abundant and diverse around fumaroles [2] [3]. Fumaroles are openings on the sides of volcanoes that emit steam and hot gases such as carbon dioxide, sulfur dioxide, hydrogen chloride and hydrogen sulfide. The steam and hot liquid from the fumaroles maintain moist hot soils and provide nutrients, which allows for microbial growth. These fumaroles also generate important physiochemical gradients since temperatures, moisture and pH decrease abruptly with distance to the fumarole.
Physiochemical Conditions
Temperatures
Air temperatures can go below - 25°C [4] and soil temperature can go up to 65°C [5]. The thermal gradients at these fumaroles are steep with temperature differences of over 60 °C within less than 0.6 m. However, beneath the surface they are also relatively sustained, allowing for a stable and diverse community structure [2].
Moisture
At warm sites water is constantly present due to the condensation of steam and the melting of snow [6]. Warmer sites tend to have higher moisture content than cooler sites [2]. However, % moisture tends to vary greatly within warm sites [2] [5].

pH
The sulfur emitted by the fumaroles generates acidic environments near the fumaroles. However, at sites adjacent to the fumaroles the high temperatures and high concentrations of CO2 help maintain a neutral pH [2].
Light
During the winter, Mount Erebus is in darkness for approximately 16 weeks [7].
Nutrients
Before reaching the surface of fumaroles, hot liquids and steam dissolve minerals from the deep surface. When they reach the surface they cause extensive soil mineralization [8]. Iron and Manganese reach toxic levels near fumaroles [2]. Carbon levels are higher closer to the fumaroles. However, both fixed carbon and nitrogen levels are low in these areas. On the other hand, these areas are rich in CO2 [2].
Community Structure
The microbial community structure is clearly dependent on physiochemical conditions, the key factors being temperature, pH and moisture [2]. The organisms that grow adjacent to fumaroles are different to those that grow further away. Not all organisms are thermophiles or psychrophiles; the temperature gradient allows for mesophiles as well [5].
The bacteria present are very diverse and there are deep branching bacterial 16S phylogenetic sequences recovered from geothermal soils community analysis [2].
A recent study hypothesizes that the microbial community at Tramway Ridge is driven by chemolithoautotrophic organisms [8].
Heterotrophic Bacillus strains have been isolated from the soil indicating the presence of algae and other autotrophic organisms that support heterotrophic growth [5].
One strain of Planctomycetes capable of anaerobic ammonia oxidation and sulfur reduction is present in considerable amounts in the geothermal soil [2].
Two cyanobacterial sequences have been identified. The cyanobacteria cannot survive the dark winters of Mt. Erebus and are thought to be involved in long-distance airborne dispersal [2][7][9].
Archea identified bare great similarity with Crenarchaeota sourced from deep subsurface environments, which are thought to play a role in chemoautotrophic nitrification [2].
Although rarely been found in the Antarctic, representatives of OP10 (hypothesized to play a role in metal cycling) have been found in Mt. Erebus whereas proteobacteria have not [2].
In addition to these organisms, 16S rRNA gene sequence analysis of a hot site (around 60 ˚C) indicates that there are several bacterial groups present that are yet to be described. The largest clade of bacteria is branched within an unknown group between candidate division OP10 and Chloroflexi [2].
Microbial adaptations
Microorganisms that live at high temperatures require adaptations such as thermo-tolerant proteins. These proteins may have a higher ion-pair content and form higher-order oligomers [10]. Membrane lipid composition is also adapted to compensate for the increased membrane fluidity induced by high temperatures [11].
Acidophiles have mechanisms to maintain an internal pH at levels close to neutral such as “unusual permeability properties, positive surface charges, high internal buffer capacity, overexpression of H+ export enzymes and unique transporters.” [2]
Significance
Extreme high-temperature environments such as those in Mount Erebus provide the conditions that are closes to those under which the first forms of life developed and thus further research could give us greater insight on the origins of life.
The openings on the Earth’s crust in combination with the extreme conditions provide an opportunity to research the subsurface microbiology. The adaptations of organism in Mt. Erebus could also have implications for the discovery of life on other planets [11].
The study of Mount Erebus could provide also examples for microbial aeolian dispersal. Further study of the cyanobacteria in the area could aid the research on this field and help explain the conditions for this mode of dispersal.
Soo et al. and Vickers have done recent research using novel approaches such as automated rRNA intergenic spacer analysis (ARISA) and restriction fragment length polymorphism (RFLP) analysis which has greatly extended the research on Mt. Erebus’s microbial diversity. This highlights the importance of using culture-independent as well as culture-dependent techniques [2] [8].
References
1. Nicolaus, B., Mariglia, F., Esposito, E., Trincone, A., Lama, L., Sharp, R., et al. (1991) Isolation of five strains of thermophilic eubacteria in Antarctica. Polar Biol 11: 425–429.
2. Soo, R., Wood, S., Grzymski, J., McDonald, I., Cary, S., (2009) Microbial biodiversity of thermophilic communities in hot mineral soils of Tramway Ridge, Mount Erebus, Antarctica. Environ Microbiol 11(3): 715–728
3. Ugolini, F.C., and Starkey, R.L. (1966) Soils and microoganisms from Mount Erebus, Antarctica. Nature 211: 440– 441.
4. Hudson, J.A., Daniel, R.M., and Morgan, H.W. (1989). Acidophilic and thermophilic Bacillus strains from geothermally heated antarctic soil. FEMS Microbiology Letters 60, 279-282.
5. Hudson, J.A., and Daniel, R.M. (1987) Enumeration of thermophilic heterotrophs in geothermally heated soils from Mount Erebus, Ross Island, Antarctica. Appl Environ Microbiol 54: 622–624.
6. Bargagli, R., Broady, P.A., and Walton, D.W.H. (1996) Pre- liminary investigation of the thermal biosystem of Mount Rittman fumaroles (northern Victoria Land, Antarctica). Antarct Sci 8: 121–126.
7. Melick, D.R., Broady, P.A., and Rowan, K.S. (1991) Mor- phological and physiological characteristics of a non- heterocystous strain of the cyanobacterium Mastigocladus laminosus Cohn from fumarolic soil on Mt Erebus, Antarc- tica. Polar Biol 11: 81–89.
8. Vickers C. J. (2012) Investigating the Physiological and Metabolic Requirements of the Tramway Ridge Microbial Community, Mt Erebus, Antarctica. M.Sc. Thesis. Unpublished.
9. Melick, D.R., Broady, P.A., and Rowan, K.S. (1991) Mor- phological and physiological characteristics of a non- heterocystous strain of the cyanobacterium Mastigocladus laminosus Cohn from fumarolic soil on Mt Erebus, Antarc- tica. Polar Biol 11: 81–89.
10. Rothschild, L.J., and Mancinelli, R.L. (2001) Life in extreme environments. Nature 409: 1092–1101.
11. Nicolaus, B., Mariglia, F., Esposito, E., Trincone, A., Lama, L., Sharp, R., et al. (1991) Isolation of five strains of ther- mophilic eubacteria in Antarctica. Polar Biol 11: 425–429.

