Mycobacterium tuberculosis review: Difference between revisions
| (86 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
[[Image: TB Culture.jpg|thumb|330px|left|Figure 1:</b> M. tuberculosis colonies.<ref name = slon2017> [https://digital.wwnorton.com/ebooks/epub/microbio4/OEBPS/Chapter03-02.xhtml Slonczewski, J., and Foster J. W.. Microbiology: An Evolving Science. New York]</ref>]] | [[Image: TB Culture.jpg|thumb|330px|left|Figure 1:</b> M. tuberculosis colonies.<ref name = slon2017> [https://digital.wwnorton.com/ebooks/epub/microbio4/OEBPS/Chapter03-02.xhtml Slonczewski, J., and Foster J. W.. Microbiology: An Evolving Science. New York]</ref>]] | ||
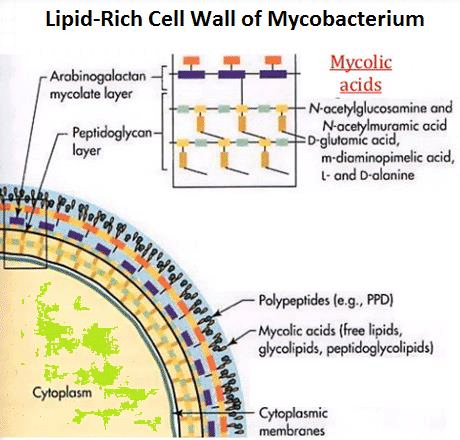
[[Image: | [[Image: Location-and-structure-of-the-Mycobacterium-tuberculosis-cord-factor.png|thumb|500px|right|<b>Figure 2:</b> Location and structure of the Mycobacterium mycolic cell wall<ref name = Saenz2015> [https://www.researchgate.net/figure/Location-and-structure-of-the-Mycobacterium-tuberculosis-cord-factor_fig2_308414494 Martynov AV, Bomko TV, Nosalskaya TN, Lisnyak Yu.V, Romanova EA, Kabluchko TV, … Farber SB. (2016). TUBERCULOSIS AS AN INFECTIOUS PATHOLOGY OF IMMUNE SYSTEM. Annals of Mechnikov Institute, (3), 8–14. http://doi.org/10.5281]</ref>]] | ||
Mycobacterium tuberculosis is a deadly human pathogen that has a staggering impact globally, causing infection disease called tuberculosis (TB). According to the Centers for Disease Control and Prevention, eight million people around the world become sick with TB and there are over two million TB-related deaths worldwide each year.<ref | Mycobacterium tuberculosis(Figure.1) is a deadly human pathogen that has a staggering impact globally, causing infection disease called tuberculosis (TB). According to the Centers for Disease Control and Prevention, eight million people around the world become sick with TB and there are over two million TB-related deaths worldwide each year.<ref>[https://cmr.asm.org/content/cmr/18/1/81.full.pdf uni Takayama,1,2,3,* Cindy Wang,1 and Gurdyal S. Besra4Clin Microbiol Rev. Pathway to Synthesis and Processing of Mycolic Acids in Mycobacterium tuberculosis 2005 Jan; 18(1): 81–101.doi: 10.1128/CMR.18.1.81-101.2005]</ref> However, most of the infection is latent and only 5 to 10% of this population has a lifetime risk of developing active tuberculosis, either within 1 or 2 years after infection (primary tuberculosis) or thereafter (secondary tuberculosis) <ref>[https://cmr.asm.org/content/15/2/294.full Reinout van Crevel, Tom H. M. Ottenhoff, Jos W. M. van der Meer,Innate Immunity to Mycobacterium tuberculosis,DOI: 10.1128/CMR.15.2.294-309.2002]</ref> | ||
Mycobacterium tuberculosis has a low generation time; cell division occurs every 18-24 hours, which is extremely slow compared with other bacteria that normally has a division rate every 20 minutes( | Mycobacterium tuberculosis is a weakly gram-positive, non-motile, rod-shaped bacterium. It is also a facultative intracellular parasite as well as an obligated aerobic. This explains why tuberculosis is a disease typically affects the lungs. Unlike other bacteria that have cell walls mainly composed of peptidoglycan, the major cell wall component of mycobacterium is lipids(Figure.2). The lipid layer makes it impervious for gram staining, and it shows either gram-positive or gram-negative. More advance methods like acid-fast staining applied to detect the function of Mycobacterium.<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4854647/ Cudahy P, Shenoi SV (April 2016). "Diagnostics for pulmonary tuberculosis". Postgraduate Medical Journal. 92 (1086): 187–93. doi:10.1136/postgradmedj-2015-133278.]</ref> | ||
Mycobacterium tuberculosis has a low generation time; cell division occurs every 18-24 hours, which is extremely slow compared with other bacteria that normally has a division rate every 20 minutes.<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4854647/ Cudahy P, Shenoi SV (April 2016). "Diagnostics for pulmonary tuberculosis". Postgraduate Medical Journal. 92 (1086): 187–93. doi:10.1136/postgradmedj-2015-133278.]</ref> The reason is that the mycolic acid has a low permeability which can protects bacterium taking damage from the immune system such as phagosome and macrophage. However, the impervious characteristic also limits nutrient accessibility, decreasing the rate of diffusion and eventually leads to a slower growth rate. | |||
==Mycobacterium tuberculosis cell wall structure== | ==Mycobacterium tuberculosis cell wall structure== | ||
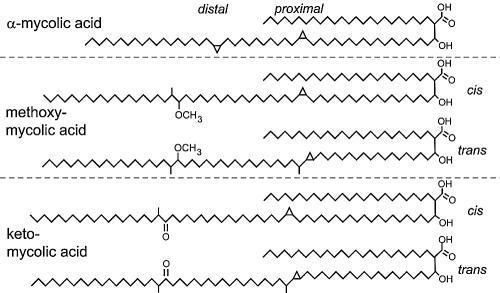
[[Image: | [[Image:Zcm0010521200001.jpg |thumb|500px|right|<b>Figure 3:</b>Chemical structures of mycolic acids from M. tuberculosis. There are five forms of mycolic acids in M. tuberculosis, illustrated with α-mycolic acid from the H37Ra strain and methoxy- and keto-mycolic acids from M. tuberculosis subsp. hominis strains DT, PN, and C. Both cyclopropane rings in α-mycolic acid have the cis configuration. The methoxy- and keto-mycolic acids can have either the cis or trans configuration on the proximal cyclopropane ring.<ref> [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC544180/ Kuni Takayama,1,2,3,* Cindy Wang,1 and Gurdyal S. Besra, Pathway to Synthesis and Processing of Mycolic Acids in Mycobacterium tuberculosis,doi: 10.1128/CMR.18.1.81-101.2005]</ref>]] | ||
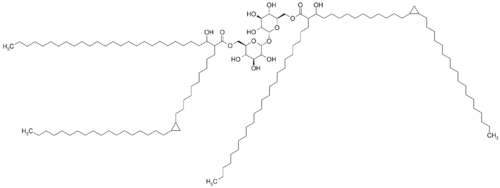
[[Image:Cord factot.png|thumb|500px|right|<b>Figure 4: </b>The general structure of Cord Factor.<ref name=sigmahopane> [https://en.wikipedia.org/wiki/Cord_factor Hopane Reference Standards for use as Petrochemical Biomarkers in Oil Field Remediation and Spill Analysis, Amann N., Sigma Aldrich, Accessed May 11th, 2018]</ref>]] | |||
[[Image: | |||
===Mycolic acid=== | |||
Mycolic acids,2-alkyl, 3-hydroxy long-chain fatty acids (FAs), are major and specific lipid components of the mycobacterial cell envelope that account for up to 60% of the whole cell dry weight. They are essential for the survival of members of the genus Mycobacterium.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/9328647 M. Daffé, P. DraperThe envelope layers of mycobacteria with reference to their pathogenicityDOI: 10.1016/s0065-2911(08)60016-8]</ref>In Mycobacterium tuberculosis cell wall, Mycolic acid can be divided into three categories: alpha-myolic acid, methoxyl-myolic acid, and ketone-myolic acid (figure.3). Alpha-mycolic acid has composed more than 70% of the mycolic acid in M. tuberculosis. It is a pure long alkyl chain attached with several cyclopropanes that contribute to the structural integrity of the cell wall complex and protect the bacillus from oxidative stress.<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC41572/ Yuan Y, Lee RE, Besra GS, Belisle JT, Barry CE 3rdProc Natl Acad Sci U S A. 1995 Jul 3; 92(14):6630-4.dentification of a gene involved in the biosynthesis of cyclopropane mycolic acids in Mycobacterium tuberculosis.doi: 10.1073/pnas.92.14.6630]</ref> Methoxyl-myolic acid made up to 10% to 15% of the mycolic acid. It has extra methoxyl group connected with fatty acid chain. The rest of the 10% to 15% mycolic acid is ketone mycolic acid which has some extra ketone groups attached on fatty acid.<ref>[https://pl.csl.sri.com/mycolate-overview.html dentification of a gene involved in the biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis.Yuan Y, Lee RE, Besra GS, Belisle JT, Barry CE 3rdProc Natl Acad Sci U S A. 1995 Jul 3; 92(14):6630-4.]</ref> Deletion of those cyclopropane rings would lead to significant attenuation in growth and deletion of the keto-mycolates would lead to restricted growth in macrophages.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/9781881 Yuan Y, Zhu Y, Crane DD, Barry CE 3rd,he effect of oxygenated mycolic acid composition on cell wall function and macrophage growth in Mycobacterium tuberculosis.dOI: 10.1046/j.1365-2958.1998.01026.x]</ref> Thus it is highly associated with virulence of Mycobacterium tuberculosis. | |||
The presence of mycolic plays a significant role in obstructing hydrophilic antimicrobials.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/9851911 Draper, P. (1998) The outer parts of the mycobacterial envelope as permeability barriers. Frontiers of Biosciences 3, 1253–1261]</ref> Because of this property, hydrophobic antibiotic like rifampicin and fluoroquinolones may be able to cross the cell wall by diffusion. However, the majority of the hydrophilic nutrients and antibiotics like isoniazid are not to diffuse through the lipid layer and they are considered to porin channel instead.<ref>[https://science.sciencemag.org/content/258/5087/1479 rias, J., Jarlier, V. and Benz, R. (1992) Porins in the cell wall of mycobacteria. Science 258, 1479–1481DOI: 10.1126/science.1279810 ]</ref> Mycobacterium tuberculosis has less abundant than other bacteria and allow a slow uptake rate of nutrient and antibiotic, making it highly resistant to all kinds of antibiotic. Besides resistance to antibiotics, hydrophobic mycolic cell wall also enables tuberculosis to survive inside the macrophage by inhibiting the action of cation proteins, lysozymes, and oxygen radicals, hiding them from the host immune system. | |||
=== | ===Cord factors=== | ||
Cord factors (trehalose 6,6′-dimycolate; TDM)is a glycolipid molecule also presence in the cell wall of mycobacterium (figure.3). It locates at the exterior parts of the cell wall and influences the arrangement of mycobacterium into long rod shape, giving it name.<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC101563/ Saita, N.; Fujiwara, N.; Yano, I.; Soejima, K.; Kobayashi, K. (1 October 2000). "Trehalose 6,6'-Dimycolate (Cord Factor) of Mycobacterium tuberculosis Induces Corneal Angiogenesis in Rats". Infection and Immunity. 68 (10): 5991–5997. doi:10.1128/IAI.68.10.5991-5997.2000. ]</ref> | |||
Cord factor is formed by a trehalose sugar and a disaccharide that esterified to two mycolic acid (Figure 4).<ref>[https://www.sciencedirect.com/science/article/pii/000630025690289X?via%3Dihub OLL, H; BLOCH, H; ASSELINEAU, J; LEDERER, E (May 1956). "The chemical structure of the cord factor of Mycobacterium tuberculosis". Biochimica et Biophysica Acta. 20 (2): 299–309. doi:10.1016/0006-3002(56)90289-x. ]</ref> One of the monosaccharides is connected with mycolic acid at its six carbon and the other monosaccharides also connected with mycolic acid at its six-carbon.<ref>[https://www.sciencedirect.com/science/article/pii/000630025690289X?via%3Dihub OLL, H; BLOCH, H; ASSELINEAU, J; LEDERER, E (May 1956). "The chemical structure of the cord factor of Mycobacterium tuberculosis". Biochimica et Biophysica Acta. 20 (2): 299–309. doi:10.1016/0006-3002(56)90289-x. ]</ref> Thus, it is named as trehalose 6,6-dimycolate. | |||
Cord factor is extremely toxic to mammalian and it can inhibit polymorphonuclear cell, a category of white blood cells with varying shape of nucleus, migration to the affected area where the infection is present.(10.5) Cord factor could also stimulate the production of TNF (tumor necrosis factors) secretion from the macrophage and T cell. This would lead to a low-grade fever in the infected individuals following by anorexia and eventually result in weight loss. Furthermore, cord factor can induce granuloma formation by its ability to stimulate cytokine production from inflammatory cells in the host.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/8306054 Perez RL, Roman J, Staton GW Jr, Hunter RL,Extravascular coagulation and fibrinolysis in murine lung inflammation induced by the mycobacterial cord factor trehalose-6,6'-dimycolate.DOI: 10.1164/ajrccm.149.2.8306054]</ref> | |||
===Wax-D=== | |||
Wax-D is a lipid complex composed by LAM, sulfatides, and glycolipids and it presents an essential role in preventing oxidation burst inside the phagosome. Sulfatides and glycolipids help mycobacterium tuberculosis in attaching to the macrophages and thusinhibiting the phagosome lysosome fusion. Wax-D also regulates internal acidity by preventing hydrogen ion entering the phagosome, ensuring its DNA replication.<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC101563/ Saita, N.; Fujiwara, N.; Yano, I.; Soejima, K.; Kobayashi, K. (1 October 2000). "Trehalose 6,6'-Dimycolate (Cord Factor) of Mycobacterium tuberculosis Induces Corneal Angiogenesis in Rats". Infection and Immunity. 68 (10): 5991–5997. doi:10.1128/IAI.68.10.5991-5997.2000. ]</ref> | |||
=== | ==Clinical behavior== | ||
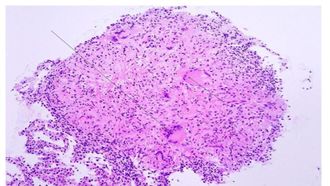
[[Image:Caseating_necrosis.jpg|thumb|330px|left|Figure 5:</b> Granuloma of Tuberculosis. Arrows pointed at multi-nucleated giant cells.<ref>[https://www.creative-biolabs.com/vaccine/mycobacterium-tuberculosis-vaccines.htm]</ref>]] | |||
[ | ===Primary infection=== | ||
Primary infection, the initial phase, occurs in people without specific immunity, generally normal children and young adults who have not previously been exposed to Mycobacterium tuberculosis.<ref name=mary>[https://www.sciencedirect.com/science/article/pii/S0379073810003567?via%3DihubJohan Dempers, M.B.Ch.B., F.C. For. Path. (S.A.), Dip. For. Med. (S.A.), CML (Unisa),a Mary Ann Sens, M.D., Ph.D.,b Shabbir Ahmed Wadee, B.Sc., M.B.Ch.B., M.Med.(For Path.), F.C. For Path.(S.A.),c Hannah C. Kinney, M.D.,d Hein J. Odendaal, M.B.Ch.B., M.Med., F.C O.G.(S.A.), F.R.C.O.G., M.D,e Colleen A. Wright, M.D., M.Med. (Anat. Path.), F.C. Path. (S.A.), F.R.C.Path., F.I.A.C., Ph.D.,f and the PASS NetworkProgressive Primary Pulmonary Tuberculosis Presenting as the Sudden Unexpected Death in Infancy: A Case Report 2010 Aug 11. doi: 10.1016/j.forsciint.2010.07.018]</ref> It occur when the inhaled bacteria enter the lung would enter the terminal air space and replicate at alveolar macrophage.<ref>[https://www.sciencedirect.com/science/article/pii/S0092867401002367?via%3Dihub Michael S. Glickman* and William R. Jacobs, Jr. Microbial Pathogenesis of Mycobacterium tuberculosis: Dawn of a Disciplinehttps://doi.org/10.1016/S0092-8674(01)00236-7 ]</ref> Some of the infected macrophage would migrate to the close lymph node, and eventually disseminate the infection to remote body parts. Primary infection can be tested by observing the present of granuloma, collection of macrophage ,at the infected area (Figure 5). Primary infection can developed into either latent infection or progressive primary infection. Latent infection take place when the M. tuberculosis have been eliminated by the innate immune system or switch into dormant stage and it is hard to differentiate those two stage based on x ray. Progressive primary infection is observed in 5-10% of patients with primary tuberculosis. Just as its name, the infection gradually worsen and worsen at the 1-2 year of the infection. This infection is developed when the immune system failed to control the out break of virus and it is usually happens at the newborn infant whose immune system is underdeveloped<ref name=mary></ref>. | |||
=== | ===secondary infection=== | ||
Secondary infection represent the reactivation of a prior latent or dormant infection and there are 5% to 10% of the secondary infection developed from the latent infection. Secondary infection could occur at any point of the host life, either in several months to decades. There are many factors that governs the occurrence of secondary infection, including the immune state of the host. When the host has experience immunity depression, their likelihood to have a secondary infection is high. Secondary infection is alway with other factors. Patients coinfected with Human immunodeficiency virus (HIV), organ transplant, and IVDU have more than 10-fold risk than the general population. Besides reactivation, secondary infection can also be caused by reinfection through bacteria outside the host. | |||
==treatment and prevention== | |||
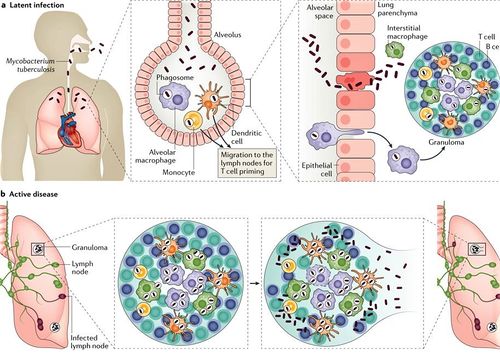
[[Image:Mycobacterium-tuberculosis-Vaccines-1.jpg|thumb|500px|right|Figure 6:</b> The Infection Mechanism of Mycobacterium tuberculosis<ref>[https://www.creative-biolabs.com/vaccine/mycobacterium-tuberculosis-vaccines.htm]</ref>]] | |||
Medical treatment of tuberculosis, together with correct diagnosis, plays an essential in the management and control of tuberculosis. <ref>[https://www.pnas.org/content/100/24/13881 Rawat R, Whitty A, Tonge PJThe isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. https://doi.org/10.1073/pnas.2235848100]</ref>Until now, there are no specific single drugs to eliminate mycobacterium tuberculosis. A combination of two stage multiple stage drug therapy is the most common method used on tuberculosis treatment. Adherence to long-term antituberculosis therapy is crucial for maintaining adequate blood drug level and drug resistance can easily evolve from mycobacterium tuberculosis if the patients apply durg inadequately <ref>[https://www.pnas.org/content/100/24/13881 Rawat R, Whitty A, Tonge PJThe isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. https://doi.org/10.1073/pnas.2235848100]</ref> | |||
The | In general, there are two different steps in the treatment of tuberculosis can be recognized, the initial (bactericidal) phase and the continuation (sterilizing) phase. The initial phase treatment usually takes place at the first two month of the treatment in which mycobacteria with a high replication rate are killed, and, consequently, with the histological pulmonary restoration and the reduction of the inflammation process, symptoms and clinical signs resolve (clinical recovery) <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4790366/ Blanchard JS, Review Molecular mechanisms of drug resistance in Mycobacterium tuberculosis doi: 10.3390/antibiotics3030317]</ref> The initial phase is also known as intensive phase as most of the drugs like , rifampin, Isoniazid, pyrazinamide, and ethambutol, are applied in the treatment. The continuation phase take place at the next four months after initial treatment. It is oriented to the elimination of semi-dormant mycobacteria. Compared with bacteria at the beginning of the therapy, semi-dormant mycobacteria has a lower replication rate and may exist a low probability of antibactericidal resistance. A patient in continuous phase looks close like a healthy individual. However, without proper drug usage like stop applying drugs, drug resistance mycobacteria may regrow, making treatment become a challenge. The drug combinations commonly used in continuous stages are rifampin and isoniazid. | ||
The mode of action of rifampicin in M. tuberculosis is by binding to the β-subunit of the RNA polymerase, inhibiting the elongation of messenger RNA <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4790366/ Blanchard JS, Review Molecular mechanisms of drug resistance in Mycobacterium tuberculosis doi: 10.3390/antibiotics3030317]</ref>isoniazid acts by inhibiting the synthesis of mycolic acids through the NADH-dependent enoyl-acyl carrier protein (ACP)-reductase, encoded by inhA <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4790366/ Blanchard JS, Review Molecular mechanisms of drug resistance in Mycobacterium tuberculosis doi: 10.3390/antibiotics3030317]</ref> | |||
[[Image: | ==Bacillus Calmette–Guérin (BCG) vaccination== | ||
[[Image: 09.jpg|thumb|330px|left|Figure 6:</b> BCG vaccine .<ref>[https://www.museumofhealthcare.ca/explore/exhibits/vaccinations/other-vaccines.html]</ref>]] | |||
===Molecular mechanism=== | |||
Until now, the only licensed vaccine against mycobacterium infection is Bacillus Calmette–Guérin (BCG) which is a lab cultured mycobacterium deriving from mycobacterium bovis(18). | |||
Unlike other mycobacterium pathogens, BCG lacks virulence as it had a deletion of the RD-1 locus encode nine genes that include a 10-kDa cultured filtered protein (CFP-10) and a 6-kDa early secreted-antigen (ESTAT-6)<ref>[https://www.tandfonline.com/doi/full/10.1586/14760584.2016.1170599 Tamara Davenne a , * and Helen McShane Why don’t we have an effective tuberculosis vaccine yet? Expert Rev Vaccines. 2016 Aug 2; 15(8): 1009–1013.Published online 2016 May 3. doi: 10.1586/14760584.2016.1170599]</ref>. These two proteins are secreted by the Snm system and both play an essential role in regulating the virulence of pathogens. ESAT-6 is capable of blocking the toll-like receptor 2 (TLR2) that is a surface protein mediating the production of cytokines necessary for the development of effective immunity. Besides, CFP/ESTAT complex was also shown to downregulate reactive oxygen species (ROS) production <ref> [https://www.ncbi.nlm.nih.gov/pubmed/17557817 de Jonge MI, Pehau-Arnaudet G, Fretz MM, et al. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol. 2007;189(16):6028–6034.DOI: 10.1128/JB.00469-07]</ref> | |||
. With the lack of expression of CFP-10 and ESAT-6, mycobacterium bovis in the BCG vaccine failed to counteract and damage the host cell system, allowing it to be killed by the immune system efficiently. | |||
===Vaccine limitation=== | |||
Although BCG is the most widely used vaccine in preventing tuberculosis in the world, it is the most controversial in current use. According to the World Health Organization, the estimation of BCG against pulmonary infection varied from 0% to 80% <ref>[https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(95)92348-9/fulltextP.E.M.Variation in protection by BCG: implications of and for heterologous immunity Communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, London https://doi.org/10.1016/S0140-6736(95)92348-9]</ref>The reason to cause this high variability is unclear but it is highly associated with genetic nutrient differences between populations, and environmental factors such as sunlight exposure, poor cold-chain maintenance, or exposure to environmental mycobacterium tuberculosis infection<ref>[https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(95)92348-9/fulltextP.E.M.Variation in protection by BCG: implications of and for heterologous immunity Communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, London https://doi.org/10.1016/S0140-6736(95)92348-9]</ref> | |||
. Therefore, the age of the person and the frequency of BCG vaccination has always varied from country to country. The world health organization is currently recommended BCG vaccination to all countries with a high incidence of TB, such as China and India. Massive immunization has never been used in low TB outbreak rate countries such as the United States<ref>[https://apps.who.int/iris/handle/10665/260307World Health Organization = Organisation mondiale de la Santé. (2018). BCG vaccines: WHO position paper – February 2018 – Vaccins BCG: Note de synthèse de l’OMS – Février 2018. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire, 93 (08), 73 - 96. World Health Organization = Organisation mondiale de la Santé.]</ref>. | |||
< | ===BCG vaccination could train the innate immune system=== | ||
Beside having high variability in against TB infection, BCG was observed to have a non-specific effect in targeting other pathogens. It was shown that macrophage pre-exposed to BCG displayed an increase in PMA-induced production of H2O2 and enhanced phagocytosis <ref>[https://www.ncbi.nlm.nih.gov/pubmed/1439583 Van ‘t Wout JW, Poell R, Van Furth R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice.Scand J Immunol. 1992;36(5):713–719 DOI: 10.1111/j.1365-3083.1992.tb03132.x]</ref>. The recent study even show that BCG is promising treatment in against COVID-19 outbreak by indirectly boost the innate immune system <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7136957/ Medical Hypotheses (nih.gov), Medical Hypotheses (6 April 2020). "Is Global BCG Vaccination Coverage Relevant to the Progression Of SARS-CoV-2 Pandemic?". PMC 7136957doi: 10.1016/j.mehy.2020.109707 </ref>. | |||
Latest revision as of 03:32, 17 May 2020
By Boyu Yang
Introduction
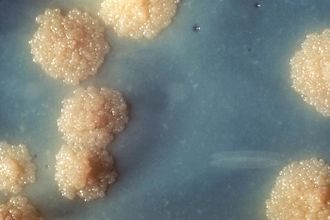
Mycobacterium tuberculosis(Figure.1) is a deadly human pathogen that has a staggering impact globally, causing infection disease called tuberculosis (TB). According to the Centers for Disease Control and Prevention, eight million people around the world become sick with TB and there are over two million TB-related deaths worldwide each year.[3] However, most of the infection is latent and only 5 to 10% of this population has a lifetime risk of developing active tuberculosis, either within 1 or 2 years after infection (primary tuberculosis) or thereafter (secondary tuberculosis) [4]
Mycobacterium tuberculosis is a weakly gram-positive, non-motile, rod-shaped bacterium. It is also a facultative intracellular parasite as well as an obligated aerobic. This explains why tuberculosis is a disease typically affects the lungs. Unlike other bacteria that have cell walls mainly composed of peptidoglycan, the major cell wall component of mycobacterium is lipids(Figure.2). The lipid layer makes it impervious for gram staining, and it shows either gram-positive or gram-negative. More advance methods like acid-fast staining applied to detect the function of Mycobacterium.[5]
Mycobacterium tuberculosis has a low generation time; cell division occurs every 18-24 hours, which is extremely slow compared with other bacteria that normally has a division rate every 20 minutes.[6] The reason is that the mycolic acid has a low permeability which can protects bacterium taking damage from the immune system such as phagosome and macrophage. However, the impervious characteristic also limits nutrient accessibility, decreasing the rate of diffusion and eventually leads to a slower growth rate.
Mycobacterium tuberculosis cell wall structure
Mycolic acid
Mycolic acids,2-alkyl, 3-hydroxy long-chain fatty acids (FAs), are major and specific lipid components of the mycobacterial cell envelope that account for up to 60% of the whole cell dry weight. They are essential for the survival of members of the genus Mycobacterium.[9]In Mycobacterium tuberculosis cell wall, Mycolic acid can be divided into three categories: alpha-myolic acid, methoxyl-myolic acid, and ketone-myolic acid (figure.3). Alpha-mycolic acid has composed more than 70% of the mycolic acid in M. tuberculosis. It is a pure long alkyl chain attached with several cyclopropanes that contribute to the structural integrity of the cell wall complex and protect the bacillus from oxidative stress.[10] Methoxyl-myolic acid made up to 10% to 15% of the mycolic acid. It has extra methoxyl group connected with fatty acid chain. The rest of the 10% to 15% mycolic acid is ketone mycolic acid which has some extra ketone groups attached on fatty acid.[11] Deletion of those cyclopropane rings would lead to significant attenuation in growth and deletion of the keto-mycolates would lead to restricted growth in macrophages.[12] Thus it is highly associated with virulence of Mycobacterium tuberculosis.
The presence of mycolic plays a significant role in obstructing hydrophilic antimicrobials.[13] Because of this property, hydrophobic antibiotic like rifampicin and fluoroquinolones may be able to cross the cell wall by diffusion. However, the majority of the hydrophilic nutrients and antibiotics like isoniazid are not to diffuse through the lipid layer and they are considered to porin channel instead.[14] Mycobacterium tuberculosis has less abundant than other bacteria and allow a slow uptake rate of nutrient and antibiotic, making it highly resistant to all kinds of antibiotic. Besides resistance to antibiotics, hydrophobic mycolic cell wall also enables tuberculosis to survive inside the macrophage by inhibiting the action of cation proteins, lysozymes, and oxygen radicals, hiding them from the host immune system.
Cord factors
Cord factors (trehalose 6,6′-dimycolate; TDM)is a glycolipid molecule also presence in the cell wall of mycobacterium (figure.3). It locates at the exterior parts of the cell wall and influences the arrangement of mycobacterium into long rod shape, giving it name.[15]
Cord factor is formed by a trehalose sugar and a disaccharide that esterified to two mycolic acid (Figure 4).[16] One of the monosaccharides is connected with mycolic acid at its six carbon and the other monosaccharides also connected with mycolic acid at its six-carbon.[17] Thus, it is named as trehalose 6,6-dimycolate.
Cord factor is extremely toxic to mammalian and it can inhibit polymorphonuclear cell, a category of white blood cells with varying shape of nucleus, migration to the affected area where the infection is present.(10.5) Cord factor could also stimulate the production of TNF (tumor necrosis factors) secretion from the macrophage and T cell. This would lead to a low-grade fever in the infected individuals following by anorexia and eventually result in weight loss. Furthermore, cord factor can induce granuloma formation by its ability to stimulate cytokine production from inflammatory cells in the host.[18]
Wax-D
Wax-D is a lipid complex composed by LAM, sulfatides, and glycolipids and it presents an essential role in preventing oxidation burst inside the phagosome. Sulfatides and glycolipids help mycobacterium tuberculosis in attaching to the macrophages and thusinhibiting the phagosome lysosome fusion. Wax-D also regulates internal acidity by preventing hydrogen ion entering the phagosome, ensuring its DNA replication.[19]
Clinical behavior
Primary infection
Primary infection, the initial phase, occurs in people without specific immunity, generally normal children and young adults who have not previously been exposed to Mycobacterium tuberculosis.[21] It occur when the inhaled bacteria enter the lung would enter the terminal air space and replicate at alveolar macrophage.[22] Some of the infected macrophage would migrate to the close lymph node, and eventually disseminate the infection to remote body parts. Primary infection can be tested by observing the present of granuloma, collection of macrophage ,at the infected area (Figure 5). Primary infection can developed into either latent infection or progressive primary infection. Latent infection take place when the M. tuberculosis have been eliminated by the innate immune system or switch into dormant stage and it is hard to differentiate those two stage based on x ray. Progressive primary infection is observed in 5-10% of patients with primary tuberculosis. Just as its name, the infection gradually worsen and worsen at the 1-2 year of the infection. This infection is developed when the immune system failed to control the out break of virus and it is usually happens at the newborn infant whose immune system is underdeveloped[21].
secondary infection
Secondary infection represent the reactivation of a prior latent or dormant infection and there are 5% to 10% of the secondary infection developed from the latent infection. Secondary infection could occur at any point of the host life, either in several months to decades. There are many factors that governs the occurrence of secondary infection, including the immune state of the host. When the host has experience immunity depression, their likelihood to have a secondary infection is high. Secondary infection is alway with other factors. Patients coinfected with Human immunodeficiency virus (HIV), organ transplant, and IVDU have more than 10-fold risk than the general population. Besides reactivation, secondary infection can also be caused by reinfection through bacteria outside the host.
treatment and prevention
Medical treatment of tuberculosis, together with correct diagnosis, plays an essential in the management and control of tuberculosis. [24]Until now, there are no specific single drugs to eliminate mycobacterium tuberculosis. A combination of two stage multiple stage drug therapy is the most common method used on tuberculosis treatment. Adherence to long-term antituberculosis therapy is crucial for maintaining adequate blood drug level and drug resistance can easily evolve from mycobacterium tuberculosis if the patients apply durg inadequately [25]
In general, there are two different steps in the treatment of tuberculosis can be recognized, the initial (bactericidal) phase and the continuation (sterilizing) phase. The initial phase treatment usually takes place at the first two month of the treatment in which mycobacteria with a high replication rate are killed, and, consequently, with the histological pulmonary restoration and the reduction of the inflammation process, symptoms and clinical signs resolve (clinical recovery) [26] The initial phase is also known as intensive phase as most of the drugs like , rifampin, Isoniazid, pyrazinamide, and ethambutol, are applied in the treatment. The continuation phase take place at the next four months after initial treatment. It is oriented to the elimination of semi-dormant mycobacteria. Compared with bacteria at the beginning of the therapy, semi-dormant mycobacteria has a lower replication rate and may exist a low probability of antibactericidal resistance. A patient in continuous phase looks close like a healthy individual. However, without proper drug usage like stop applying drugs, drug resistance mycobacteria may regrow, making treatment become a challenge. The drug combinations commonly used in continuous stages are rifampin and isoniazid. The mode of action of rifampicin in M. tuberculosis is by binding to the β-subunit of the RNA polymerase, inhibiting the elongation of messenger RNA [27]isoniazid acts by inhibiting the synthesis of mycolic acids through the NADH-dependent enoyl-acyl carrier protein (ACP)-reductase, encoded by inhA [28]
Bacillus Calmette–Guérin (BCG) vaccination
Molecular mechanism
Until now, the only licensed vaccine against mycobacterium infection is Bacillus Calmette–Guérin (BCG) which is a lab cultured mycobacterium deriving from mycobacterium bovis(18). Unlike other mycobacterium pathogens, BCG lacks virulence as it had a deletion of the RD-1 locus encode nine genes that include a 10-kDa cultured filtered protein (CFP-10) and a 6-kDa early secreted-antigen (ESTAT-6)[30]. These two proteins are secreted by the Snm system and both play an essential role in regulating the virulence of pathogens. ESAT-6 is capable of blocking the toll-like receptor 2 (TLR2) that is a surface protein mediating the production of cytokines necessary for the development of effective immunity. Besides, CFP/ESTAT complex was also shown to downregulate reactive oxygen species (ROS) production [31] . With the lack of expression of CFP-10 and ESAT-6, mycobacterium bovis in the BCG vaccine failed to counteract and damage the host cell system, allowing it to be killed by the immune system efficiently.
Vaccine limitation
Although BCG is the most widely used vaccine in preventing tuberculosis in the world, it is the most controversial in current use. According to the World Health Organization, the estimation of BCG against pulmonary infection varied from 0% to 80% [32]The reason to cause this high variability is unclear but it is highly associated with genetic nutrient differences between populations, and environmental factors such as sunlight exposure, poor cold-chain maintenance, or exposure to environmental mycobacterium tuberculosis infection[33] . Therefore, the age of the person and the frequency of BCG vaccination has always varied from country to country. The world health organization is currently recommended BCG vaccination to all countries with a high incidence of TB, such as China and India. Massive immunization has never been used in low TB outbreak rate countries such as the United States[34].
BCG vaccination could train the innate immune system
Beside having high variability in against TB infection, BCG was observed to have a non-specific effect in targeting other pathogens. It was shown that macrophage pre-exposed to BCG displayed an increase in PMA-induced production of H2O2 and enhanced phagocytosis [35]. The recent study even show that BCG is promising treatment in against COVID-19 outbreak by indirectly boost the innate immune system [36].
- ↑ Slonczewski, J., and Foster J. W.. Microbiology: An Evolving Science. New York
- ↑ Martynov AV, Bomko TV, Nosalskaya TN, Lisnyak Yu.V, Romanova EA, Kabluchko TV, … Farber SB. (2016). TUBERCULOSIS AS AN INFECTIOUS PATHOLOGY OF IMMUNE SYSTEM. Annals of Mechnikov Institute, (3), 8–14. http://doi.org/10.5281
- ↑ uni Takayama,1,2,3,* Cindy Wang,1 and Gurdyal S. Besra4Clin Microbiol Rev. Pathway to Synthesis and Processing of Mycolic Acids in Mycobacterium tuberculosis 2005 Jan; 18(1): 81–101.doi: 10.1128/CMR.18.1.81-101.2005
- ↑ Reinout van Crevel, Tom H. M. Ottenhoff, Jos W. M. van der Meer,Innate Immunity to Mycobacterium tuberculosis,DOI: 10.1128/CMR.15.2.294-309.2002
- ↑ Cudahy P, Shenoi SV (April 2016). "Diagnostics for pulmonary tuberculosis". Postgraduate Medical Journal. 92 (1086): 187–93. doi:10.1136/postgradmedj-2015-133278.
- ↑ Cudahy P, Shenoi SV (April 2016). "Diagnostics for pulmonary tuberculosis". Postgraduate Medical Journal. 92 (1086): 187–93. doi:10.1136/postgradmedj-2015-133278.
- ↑ Kuni Takayama,1,2,3,* Cindy Wang,1 and Gurdyal S. Besra, Pathway to Synthesis and Processing of Mycolic Acids in Mycobacterium tuberculosis,doi: 10.1128/CMR.18.1.81-101.2005
- ↑ Hopane Reference Standards for use as Petrochemical Biomarkers in Oil Field Remediation and Spill Analysis, Amann N., Sigma Aldrich, Accessed May 11th, 2018
- ↑ M. Daffé, P. DraperThe envelope layers of mycobacteria with reference to their pathogenicityDOI: 10.1016/s0065-2911(08)60016-8
- ↑ Yuan Y, Lee RE, Besra GS, Belisle JT, Barry CE 3rdProc Natl Acad Sci U S A. 1995 Jul 3; 92(14):6630-4.dentification of a gene involved in the biosynthesis of cyclopropane mycolic acids in Mycobacterium tuberculosis.doi: 10.1073/pnas.92.14.6630
- ↑ dentification of a gene involved in the biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis.Yuan Y, Lee RE, Besra GS, Belisle JT, Barry CE 3rdProc Natl Acad Sci U S A. 1995 Jul 3; 92(14):6630-4.
- ↑ Yuan Y, Zhu Y, Crane DD, Barry CE 3rd,he effect of oxygenated mycolic acid composition on cell wall function and macrophage growth in Mycobacterium tuberculosis.dOI: 10.1046/j.1365-2958.1998.01026.x
- ↑ Draper, P. (1998) The outer parts of the mycobacterial envelope as permeability barriers. Frontiers of Biosciences 3, 1253–1261
- ↑ rias, J., Jarlier, V. and Benz, R. (1992) Porins in the cell wall of mycobacteria. Science 258, 1479–1481DOI: 10.1126/science.1279810
- ↑ Saita, N.; Fujiwara, N.; Yano, I.; Soejima, K.; Kobayashi, K. (1 October 2000). "Trehalose 6,6'-Dimycolate (Cord Factor) of Mycobacterium tuberculosis Induces Corneal Angiogenesis in Rats". Infection and Immunity. 68 (10): 5991–5997. doi:10.1128/IAI.68.10.5991-5997.2000.
- ↑ OLL, H; BLOCH, H; ASSELINEAU, J; LEDERER, E (May 1956). "The chemical structure of the cord factor of Mycobacterium tuberculosis". Biochimica et Biophysica Acta. 20 (2): 299–309. doi:10.1016/0006-3002(56)90289-x.
- ↑ OLL, H; BLOCH, H; ASSELINEAU, J; LEDERER, E (May 1956). "The chemical structure of the cord factor of Mycobacterium tuberculosis". Biochimica et Biophysica Acta. 20 (2): 299–309. doi:10.1016/0006-3002(56)90289-x.
- ↑ Perez RL, Roman J, Staton GW Jr, Hunter RL,Extravascular coagulation and fibrinolysis in murine lung inflammation induced by the mycobacterial cord factor trehalose-6,6'-dimycolate.DOI: 10.1164/ajrccm.149.2.8306054
- ↑ Saita, N.; Fujiwara, N.; Yano, I.; Soejima, K.; Kobayashi, K. (1 October 2000). "Trehalose 6,6'-Dimycolate (Cord Factor) of Mycobacterium tuberculosis Induces Corneal Angiogenesis in Rats". Infection and Immunity. 68 (10): 5991–5997. doi:10.1128/IAI.68.10.5991-5997.2000.
- ↑ [1]
- ↑ 21.0 21.1 Dempers, M.B.Ch.B., F.C. For. Path. (S.A.), Dip. For. Med. (S.A.), CML (Unisa),a Mary Ann Sens, M.D., Ph.D.,b Shabbir Ahmed Wadee, B.Sc., M.B.Ch.B., M.Med.(For Path.), F.C. For Path.(S.A.),c Hannah C. Kinney, M.D.,d Hein J. Odendaal, M.B.Ch.B., M.Med., F.C O.G.(S.A.), F.R.C.O.G., M.D,e Colleen A. Wright, M.D., M.Med. (Anat. Path.), F.C. Path. (S.A.), F.R.C.Path., F.I.A.C., Ph.D.,f and the PASS NetworkProgressive Primary Pulmonary Tuberculosis Presenting as the Sudden Unexpected Death in Infancy: A Case Report 2010 Aug 11. doi: 10.1016/j.forsciint.2010.07.018
- ↑ Michael S. Glickman* and William R. Jacobs, Jr. Microbial Pathogenesis of Mycobacterium tuberculosis: Dawn of a Disciplinehttps://doi.org/10.1016/S0092-8674(01)00236-7
- ↑ [2]
- ↑ Rawat R, Whitty A, Tonge PJThe isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. https://doi.org/10.1073/pnas.2235848100
- ↑ Rawat R, Whitty A, Tonge PJThe isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. https://doi.org/10.1073/pnas.2235848100
- ↑ Blanchard JS, Review Molecular mechanisms of drug resistance in Mycobacterium tuberculosis doi: 10.3390/antibiotics3030317
- ↑ Blanchard JS, Review Molecular mechanisms of drug resistance in Mycobacterium tuberculosis doi: 10.3390/antibiotics3030317
- ↑ Blanchard JS, Review Molecular mechanisms of drug resistance in Mycobacterium tuberculosis doi: 10.3390/antibiotics3030317
- ↑ [3]
- ↑ Tamara Davenne a , * and Helen McShane Why don’t we have an effective tuberculosis vaccine yet? Expert Rev Vaccines. 2016 Aug 2; 15(8): 1009–1013.Published online 2016 May 3. doi: 10.1586/14760584.2016.1170599
- ↑ de Jonge MI, Pehau-Arnaudet G, Fretz MM, et al. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol. 2007;189(16):6028–6034.DOI: 10.1128/JB.00469-07
- ↑ in protection by BCG: implications of and for heterologous immunity Communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, London https://doi.org/10.1016/S0140-6736(95)92348-9
- ↑ in protection by BCG: implications of and for heterologous immunity Communicable Disease Epidemiology Unit, London School of Hygiene and Tropical Medicine, London https://doi.org/10.1016/S0140-6736(95)92348-9
- ↑ Health Organization = Organisation mondiale de la Santé. (2018). BCG vaccines: WHO position paper – February 2018 – Vaccins BCG: Note de synthèse de l’OMS – Février 2018. Weekly Epidemiological Record = Relevé épidémiologique hebdomadaire, 93 (08), 73 - 96. World Health Organization = Organisation mondiale de la Santé.
- ↑ Van ‘t Wout JW, Poell R, Van Furth R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice.Scand J Immunol. 1992;36(5):713–719 DOI: 10.1111/j.1365-3083.1992.tb03132.x
- ↑ [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7136957/ Medical Hypotheses (nih.gov), Medical Hypotheses (6 April 2020). "Is Global BCG Vaccination Coverage Relevant to the Progression Of SARS-CoV-2 Pandemic?". PMC 7136957doi: 10.1016/j.mehy.2020.109707
