Mycoplasma haemocanis: Difference between revisions
| (15 intermediate revisions by the same user not shown) | |||
| Line 29: | Line 29: | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
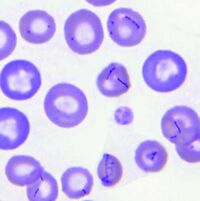
''Mycoplasma haemocanis'' features a relatively simple cell structure, lacking a cell wall. ''M. haemocanis'' most commonly appears morphologically as separate chains of cocci that are arranged linearly and adhere to the surface of red blood cells | ''Mycoplasma haemocanis'' features a relatively simple cell structure, lacking a cell wall. ''M. haemocanis'' most commonly appears morphologically as separate chains of cocci that are arranged linearly and adhere to the surface of red blood cells [7]. It can also appear as rod or ring-like structures. The absence of a cell wall causes the bacteria to stain gram negative. | ||
It is a hemotrophic bacteria that relies on the host for the nutrients required for survival. The bacteria itself, like most hemoplasma, lacks the metabolic pathways that would be required for the production of energy as well as the synthesis of fundamental cellular components. This is likely a result of its adaptation to the blood habitat which is very nutrient rich | It is a hemotrophic bacteria that relies on the host for the nutrients required for survival. The bacteria itself, like most hemoplasma, lacks the metabolic pathways that would be required for the production of energy as well as the synthesis of fundamental cellular components[6]. This is likely a result of its adaptation to the blood habitat which is very nutrient rich [2]. ''M. haemocanis'' likely relies on metabolic pathways similar to the ones that M.haemofelis, its feline infecting counterpart, does as it features orthologs for all of the coding sequences identified in M. haemofelis [2]. | ||
The bacteria is entirely dependent on erythrocytes and their growth factors but the exact methodology behind this is still widely unknown | The bacteria is entirely dependent on erythrocytes and their growth factors but the exact methodology behind this is still widely unknown [6]. ''Mycoplasma haemocanis'' is able to thrive from exploiting the metabolism of red blood cells and garnering their nutrients; however, this comes at a cost to the erythrocytes themselves. The red blood cells experience a shortened lifespan and more intense anemia symptoms in the presence of ''M.haemocanis'' [2]. | ||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
''Mycoplasma haemocanis'' resides on the surface of its canine hosts' erythrocytes. It adheres to the cells' surfaces [3]. It has been proposed that ''M. haemocanis'' may rely on the use of antigenic variation as a key for proliferation [2]. This bacteria causes overt hemolytic anemia in immunocompromised and splenectomized canines as the bacteria causes the red blood cells to lyse faster than new ones can be produced . In dogs with healthy immune systems the bacteria is typically asymptomatic. Hemolytic anemia symptoms in canines include but are not limited to weakness, pale gums, rapid breathing, lethargy, and lack of appetite [11]. ''M. haemocanis'' in canines is commonly diagnosed via PCR treatment and doxycycline is the typical form of treatment[8]. It has a prevalence as high as 40% in some parts of the world [3]. The mode of transmission for mycoplasma haemocanis has yet to be formally elucidated; however various studies have been done on the matter [1].The transfer of infected blood in clinical settings, reliance on arthropod vectors and aggressive canine interactions have been proposed as possible modes of transmission [3,9]. A study conducted in Brazil pointed to, commonly known as the brown dog tick, ''Rhipicephalus sanguineus'' as the vector responsible for the transmission of the bacteria in dogs[9]. A study in Chile pointed to aggressive interactions amongst male dogs as being the most plausible mode of transmission and noted higher rates of the bacteria in places the brown dog tick could not live [10]. Further studies will need to be done to fully determine the transmission of ''M.haemocanis''. | |||
==References== | ==References== | ||
| Line 48: | Line 48: | ||
[5] U.S. National Library of Medicine. (n.d.). Mycoplasma Haemocanis - NCBI - NLM. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/datasets/taxonomy/136241/ | [5] U.S. National Library of Medicine. (n.d.). Mycoplasma Haemocanis - NCBI - NLM. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/datasets/taxonomy/136241/ | ||
[6] Millán J, Di Cataldo S, Volokhov DV, Becker DJ. Worldwide occurrence of haemoplasmas in wildlife: Insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound Emerg Dis. 2021; 68: 3236–3256. https://doi.org/10.1111/tbed.13932 | |||
[7] Mark S. Thompson, Part Three - Laboratory Values and Interpretation of Results, | |||
Editor(s): Mark S. Thompson, Small Animal Medical Differential Diagnosis (Third Edition),W.B. Saunders, 2018, Pages 307-353, ISBN 9780323498302, https://doi.org/10.1016/B978-0-323-49830-2.00003-2. | |||
[8] Biondo, A. W., Dos Santos, A. P., Guimarães, A. M., Vieira, R. F., Vidotto, O., Macieira, D.deB., Almosny, N. R., Molento, M. B., Timenetsky, J., de Morais, H. A., González, F. H., & Messick, J. B. (2009). A review of the occurrence of hemoplasmas (hemotrophic mycoplasmas) in Brazil. Revista brasileira de parasitologia veterinaria = Brazilian journal of veterinary parasitology : Orgao Oficial do Colegio Brasileiro de Parasitologia Veterinaria, 18(3), 1–7. https://doi.org/10.4322/rbpv.01803001 | |||
[9] Seneviratna, P., Weerasinghe, & Ariyadasa, S. (1973). Transmission of Haemobartonella canis by the dog tick, Rhipicephalus sanguineus. Research in veterinary science, 14(1), 112–114. | |||
[10] Di Cataldo, S., Cevidanes, A., Ulloa-Contreras, C., Sacristán, I., Peñaloza-Madrid, D., Vianna, J., González-Acuña, D., Sallaberry-Pincheira, N., Cabello, J., Napolitano, C., Hidalgo-Hermoso, E., Acosta-Jamett, G., & Millán, J. (2021). Widespread infection with hemotropic Mycoplasmas in free-ranging dogs and wild foxes across six bioclimatic regions of Chile. Microorganisms, 9(5), 919. https://doi.org/10.3390/microorganisms9050919 | |||
[11]Brennan, K. (2018, February 16). Immune mediated disease in dogs: Canna-pet®. Canna. https://canna-pet.com/articles/immune-mediated-disease-dogs/ | |||
==Author== | ==Author== | ||
Latest revision as of 03:21, 26 April 2024
Classification
Bacteria; Mycoplasmatota; Mollicutes; Mycoplasmatales; Mycoplasmataceae [5]
Species
|
NCBI: Taxonomy |
Mycoplasma haemocanis
Description and Significance
Mycoplasma haemocanis is a hemotrophic, gram-negative bacterial species apart of the Mycoplasma genus. The species and genus is characterized by its lack of a cell wall, lack of flagella, and its small size of less than 1 µm. Formerly known as Haemobartonella canis, this species was first discovered in Germany in 1928. It was later reclassified as a hemotrophic mycoplasma due to the similarity of its 16s ribosomal RNA (rRNA) gene and the 16s rRNA sequence of the Mycoplasma genus [4]. This species is commonly associated with causing hemolytic anemia in immunocompromised canines, specifically those who have undergone a splenectomy [1]. M. haemocanis attaches to the surface of erythrocytes (red blood cells) in hosts and can be visualized in a blood smear either as single coccoid cells or "bowed-looking" chains. This species is uncultivable in vitro, and instead depends on a host cell for survival [2,4]. The bacteria has a similar resemblance to the feline equivalent Mycoplasma haemofelis, which causes hemolytic anemia in cats. In a genetic comparison of the species' genomes, the two are actually concluded to be two different species infecting two distinct animals [2,3].
Genome Structure
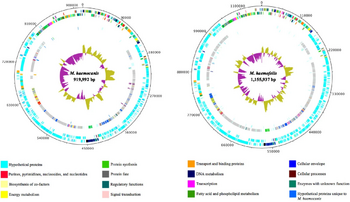
In 2012, one strain of M. haemocanis (Illinois) was isolated from the blood of an infected dog, then fully sequenced. The genome of the strain contains a singular circular chromosome comprising of 919,992 base pairs [2]. Compared to the average Mycoplasma genome size range of 580-2000 kb, the genome of M. haemocanis is consistent among other species in the same genus [4]. The genome also contains a low G+C (guanine and cytosine) content of 35%, which is characteristic for mycoplasmal species. Researchers discovered the presence of 1173 unique coding sequences (CDS), with around 75% coding for hypothetical proteins. Genome analyses of the CDS showed that 35% of the sequences are associated with the plasma membrane [2].
In comparing the genomes of M. haemocanis and M. haemofelis, analysis of the 16s rRNA sequence is not sufficient enough to distinguish whether the two species are different. Before 2012, the only genotypic evidence for the bacteria being separate species was the difference in RNase P gene sequences. Since the complete genome sequencing of the Illinois strain, the full sequence analysis including average nucleotide identity (ANI;85%) and tetranucleotides (0.95) has fallen below the cutoff value for being the same species [2].
Cell Structure, Metabolism and Life Cycle
Mycoplasma haemocanis features a relatively simple cell structure, lacking a cell wall. M. haemocanis most commonly appears morphologically as separate chains of cocci that are arranged linearly and adhere to the surface of red blood cells [7]. It can also appear as rod or ring-like structures. The absence of a cell wall causes the bacteria to stain gram negative.
It is a hemotrophic bacteria that relies on the host for the nutrients required for survival. The bacteria itself, like most hemoplasma, lacks the metabolic pathways that would be required for the production of energy as well as the synthesis of fundamental cellular components[6]. This is likely a result of its adaptation to the blood habitat which is very nutrient rich [2]. M. haemocanis likely relies on metabolic pathways similar to the ones that M.haemofelis, its feline infecting counterpart, does as it features orthologs for all of the coding sequences identified in M. haemofelis [2]. The bacteria is entirely dependent on erythrocytes and their growth factors but the exact methodology behind this is still widely unknown [6]. Mycoplasma haemocanis is able to thrive from exploiting the metabolism of red blood cells and garnering their nutrients; however, this comes at a cost to the erythrocytes themselves. The red blood cells experience a shortened lifespan and more intense anemia symptoms in the presence of M.haemocanis [2].
Ecology and Pathogenesis
Mycoplasma haemocanis resides on the surface of its canine hosts' erythrocytes. It adheres to the cells' surfaces [3]. It has been proposed that M. haemocanis may rely on the use of antigenic variation as a key for proliferation [2]. This bacteria causes overt hemolytic anemia in immunocompromised and splenectomized canines as the bacteria causes the red blood cells to lyse faster than new ones can be produced . In dogs with healthy immune systems the bacteria is typically asymptomatic. Hemolytic anemia symptoms in canines include but are not limited to weakness, pale gums, rapid breathing, lethargy, and lack of appetite [11]. M. haemocanis in canines is commonly diagnosed via PCR treatment and doxycycline is the typical form of treatment[8]. It has a prevalence as high as 40% in some parts of the world [3]. The mode of transmission for mycoplasma haemocanis has yet to be formally elucidated; however various studies have been done on the matter [1].The transfer of infected blood in clinical settings, reliance on arthropod vectors and aggressive canine interactions have been proposed as possible modes of transmission [3,9]. A study conducted in Brazil pointed to, commonly known as the brown dog tick, Rhipicephalus sanguineus as the vector responsible for the transmission of the bacteria in dogs[9]. A study in Chile pointed to aggressive interactions amongst male dogs as being the most plausible mode of transmission and noted higher rates of the bacteria in places the brown dog tick could not live [10]. Further studies will need to be done to fully determine the transmission of M.haemocanis.
References
[1] Willi, B., Novacco, M., Meli, M., Wolf-Jäckel, G., Boretti, F., Wengi, N., Lutz, H., & Hofmann-Lehmann, R. (2010). Haemotropic mycoplasmas of cats and dogs: transmission, diagnosis, prevalence and importance in Europe. Schweizer Archiv fur Tierheilkunde, 152(5), 237–244. https://doi.org/10.1024/0036-7281/a000055
[2] do Nascimento, N.C., Santos, A.P., Guimaraes, A.M. et al. (2012). Mycoplasma haemocanis – the canine hemoplasma and its feline counterpart in the genomic era. Veterinary Research, 43(1), 66. https://doi.org/10.1186/1297-9716-43-66
[3] Hemotropic Mycoplasma infections in animals - circulatory system. Merck Veterinary Manual. (n.d.). https://www.merckvetmanual.com/circulatory-system/blood-parasites/hemotropic-mycoplasma-infections-in-animals
[4] Messick, J. B. (2004). Hemotrophic mycoplasmas (hemoplasmas): A review and new insights into pathogenic potential. Veterinary Clinical Pathology, 33(1), 2–13. https://doi.org/10.1111/j.1939-165x.2004.tb00342.x
[5] U.S. National Library of Medicine. (n.d.). Mycoplasma Haemocanis - NCBI - NLM. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/datasets/taxonomy/136241/
[6] Millán J, Di Cataldo S, Volokhov DV, Becker DJ. Worldwide occurrence of haemoplasmas in wildlife: Insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound Emerg Dis. 2021; 68: 3236–3256. https://doi.org/10.1111/tbed.13932
[7] Mark S. Thompson, Part Three - Laboratory Values and Interpretation of Results, Editor(s): Mark S. Thompson, Small Animal Medical Differential Diagnosis (Third Edition),W.B. Saunders, 2018, Pages 307-353, ISBN 9780323498302, https://doi.org/10.1016/B978-0-323-49830-2.00003-2.
[8] Biondo, A. W., Dos Santos, A. P., Guimarães, A. M., Vieira, R. F., Vidotto, O., Macieira, D.deB., Almosny, N. R., Molento, M. B., Timenetsky, J., de Morais, H. A., González, F. H., & Messick, J. B. (2009). A review of the occurrence of hemoplasmas (hemotrophic mycoplasmas) in Brazil. Revista brasileira de parasitologia veterinaria = Brazilian journal of veterinary parasitology : Orgao Oficial do Colegio Brasileiro de Parasitologia Veterinaria, 18(3), 1–7. https://doi.org/10.4322/rbpv.01803001
[9] Seneviratna, P., Weerasinghe, & Ariyadasa, S. (1973). Transmission of Haemobartonella canis by the dog tick, Rhipicephalus sanguineus. Research in veterinary science, 14(1), 112–114.
[10] Di Cataldo, S., Cevidanes, A., Ulloa-Contreras, C., Sacristán, I., Peñaloza-Madrid, D., Vianna, J., González-Acuña, D., Sallaberry-Pincheira, N., Cabello, J., Napolitano, C., Hidalgo-Hermoso, E., Acosta-Jamett, G., & Millán, J. (2021). Widespread infection with hemotropic Mycoplasmas in free-ranging dogs and wild foxes across six bioclimatic regions of Chile. Microorganisms, 9(5), 919. https://doi.org/10.3390/microorganisms9050919
[11]Brennan, K. (2018, February 16). Immune mediated disease in dogs: Canna-pet®. Canna. https://canna-pet.com/articles/immune-mediated-disease-dogs/
Author
Page authored by Erin Byers & Olivia Choros, students of Prof. Jay Lennon at IndianaUniversity.