Natural Killer Cell: Difference between revisions
No edit summary |
|||
| (159 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Overview== | ==Overview== | ||
[[Image:Nk.cells.LM.EM.jpg|thumb|300px|right|Natural killer cells (NK cells) under light microscopy (A) and electron microscopy (B). In <i> | [[Image:Nk.cells.LM.EM.jpg|thumb|300px|right|<b>Figure 1:</b> Natural killer cells (NK cells) under light microscopy (A) and electron microscopy (B). In <i>Tsuchiyama J. et al. 1998</i>. Link:https://ashpublications.org/blood/article/92/4/1374/247309/Characterization-of-a-Novel-Human-Natural-Killer]] | ||
Natural killer cells (NK cells) are a type of granular cytotoxic lymphocytes that are non-adherent and non-phagocytic. NK cells were originally defined as a subset of lymphocytes that have natural cytotoxic activity against certain types of tumorous cells and endogenous type-C viruses in mice. Natural cytotoxicity refers to the fact that they can rapidly cause tumor cells’ lyses in the absence of any previous stimulation <ref>Herberman, R. B., Nunn, M. E., Holden, H. T. and Lavrin, D. H. (1975), Natural cytotoxic rectivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer, 16: 230-239. doi:10.1002/ijc.2910160205</ref>,<ref>Herberman, R. B., Nunn, M. E. and Lavrin, D. H. (1975), Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer, 16: 216-229. doi:10.1002/ijc.2910160204</ref>. They were first named in an article in 1976 <ref>WOLFE, S., TRACEY, D. & HENNEY, C. Induction of “natural killer” cells by BCG. Nature 262, 584–586 (1976) doi:10.1038/262584a0</ref> and later categorized as part of the innate immune system due to their morphology, origin (bone marrow), and lack of antigen-specific receptors (such as those on T and B-cells’ surfaces) and their respective genes.<ref>Eidenschenk, C., Dunne, J., Jouanguy, E., Fourlinnie, C., Gineau, L., Bacq, D., … Feighery, C. (2006). A Novel Primary Immunodeficiency with Specific Natural-Killer Cell Deficiency Maps to the Centromeric Region of Chromosome 8. The American Journal of Human Genetics, 78(4), 721–727. doi: 10.1086/503269</ref>,<ref>Trinchieri, G. Biology of natural | Natural killer cells (NK cells) are a type of granular cytotoxic lymphocytes that are non-adherent and non-phagocytic. NK cells were originally defined as a subset of lymphocytes that have natural cytotoxic activity against certain types of tumorous cells and endogenous type-C viruses in mice. Natural cytotoxicity refers to the fact that they can rapidly cause tumor cells’ lyses in the absence of any previous stimulation <ref name = Herberman1>Herberman, R. B., Nunn, M. E., Holden, H. T. and Lavrin, D. H. (1975), Natural cytotoxic rectivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer, 16: 230-239. doi:10.1002/ijc.2910160205</ref>,<ref name = Herberman2>Herberman, R. B., Nunn, M. E. and Lavrin, D. H. (1975), Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer, 16: 216-229. doi:10.1002/ijc.2910160204</ref>. They were first named in an article in 1976 <ref name = Wolfe>WOLFE, S., TRACEY, D. & HENNEY, C. Induction of “natural killer” cells by BCG. Nature 262, 584–586 (1976) doi:10.1038/262584a0</ref> and later categorized as part of the innate immune system due to their morphology, origin (bone marrow), and lack of antigen-specific receptors (such as those on T and B-cells’ surfaces) and their respective genes.<ref name = Eidenschenk>Eidenschenk, C., Dunne, J., Jouanguy, E., Fourlinnie, C., Gineau, L., Bacq, D., … Feighery, C. (2006). A Novel Primary Immunodeficiency with Specific Natural-Killer Cell Deficiency Maps to the Centromeric Region of Chromosome 8. The American Journal of Human Genetics, 78(4), 721–727. doi: 10.1086/503269</ref>,<ref name = Trinchieri>Trinchieri, G. Biology of natural killer cells. Adv. Immunology. Volume 47, 187-376 (1989). doi: 10.1016/S0065-2776(08)60664-1</ref> | ||
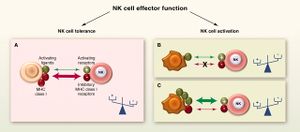
[[Image:NK.cell.immune.regulation.png|thumb|300px|left|<b>Figure 2:</b> Regulation of immune responses by NK cells. In <i>Functions of Natural Killer cells (Vivier et al. 2008)</i>. Link:https://www.nature.com/articles/ni1582]][[Image:NK.cell.effector.function.jpg|thumb|300px|right|<b>Figure 3:</b> Cytotoxic function of NK cells. In <i>Innate or Adaptive Immunity? The Example of Natural Killer Cells (Vivier et al. 2011)</i>. Link: https://science.sciencemag.org/content/331/6013/44/F3]] | |||
NK cells share many features with leukocytes of the innate immune system, such as: granular cytoplasm, spontaneous activity, and susceptibility to positive regulation by immune stimuli (dendritic cells’ cytokinin).<ref>Trinchieri, G. Biology of natural keller cells. Adv. Immunology. Volume 47, 187-376 (1989). doi: 10.1016/S0065-2776(08)60664-1</ref> However, research has shown that NK cells can retain antigen-specific immunological memory <ref>Sun, J., Beilke, J. & Lanier, L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009) doi:10.1038/nature07665</ref>, characteristics common to T and B-cells of the adaptive immune system, and interact with T-cells and macrophages to control immune response <ref>Raulet, D. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol 5, 996–1002 (2004) doi:10.1038/ni1114</ref>,<ref>Dommelen, S. L. V., Sumaria, N., Schreiber, R. D., Scalzo, A. A., Smyth, M. J., & Degli-Esposti, M. A. (2006). Perforin and Granzymes Have Distinct Roles in Defensive Immunity and Immunopathology. Immunity, 25(5), 835–848. doi: 10.1016/j.immuni.2006.09.010</ref>, a role which is usually associated with regulatory T-cells. Furthermore, studies into the immunological reactions against cytomegalovirus in mice and human has generated evidences that certain subsets of NK cells can be activated and stimulated to multiply in response to specific pathogens.<ref>Dokun, A. O., Kim, S., Smith, H. R., Kang, H.-S. P., Chu, D. T., & Yokoyama, W. M. (2001). Specific and nonspecific NK cell activation during virus infection. Nature Immunology, 2(10), 951–956. doi: 10.1038/ni714</ref>,<ref>Walter, L. (2011). Faculty of 1000 evaluation for Expansion of a unique CD57⁺NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. F1000 - Post-Publication Peer Review of the Biomedical Literature. doi: 10.3410/f.12631956.13874054</ref> Thus, NK cells are now considered to be a conjunction point of both the innate and adaptive immune systems. | |||
NK cells share a similar cytolytic mechanism of granule-exocytosis<ref>Henkart M.P., Henkart P.A. (1982) Lymphocyte Mediated Cytolysis as a Secretory Phenomenon. In: Clark W.R., Golstein P. (eds) Mechanisms of Cell-Mediated Cytotoxicity. Advances in Experimental Medicine and Biology, vol 146. Springer, Boston, MA</ref> with killer T cells where they utilize the two crucial cytolytic enzyme: perforin and granzyme.<ref>Dommelen, S. L. V., Sumaria, N., Schreiber, R. D., Scalzo, A. A., Smyth, M. J., & Degli-Esposti, M. A. (2006). Perforin and Granzymes Have Distinct Roles in Defensive Immunity and Immunopathology. Immunity, 25(5), 835–848. doi: 10.1016/j.immuni.2006.09.010</ref>,<ref>Lowin, B., Beermann, F., Schmidt, A., & Tschopp, J. (1994). A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proceedings of the National Academy of Sciences, 91(24), 11571–11575. doi: 10.1073/pnas.91.24.11571</ref>,<ref>Mahrus, S., & Craik, C. S. (2005). Selective Chemical Functional Probes of Granzymes A and B Reveal Granzyme B Is a Major Effector of Natural Killer Cell-Mediated Lysis of Target Cells. Chemistry & Biology, 12(5), 567–577. doi: 10.1016/j.chembiol.2005.03.006</ref> Moreover, NK cells also induce apoptosis in target cells by the Fas-mediated pathway using the Fas ligands.<ref>Oshimi, Y., Oda, S., Honda, Y., Nagata, S., & Miyazaki, S. (n.d.). Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. The Journal of Immunology, 157(7), 2909–2915</ref> However, unlike T and B cells of the adaptive immune system, NK cells do not rely on antigen presentation by the major histocompatibility complex (MHC) class I on cells’ membranes to be activated, rather, they utilize a combination of membranal markers to distinguish between the “self” and “non-self” (or “infected self”).<ref>Vivier, E., Tomasello, E., Baratin, M., Walzer, T., & Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), 503–510. Retrieved from: https://doi-org.libproxy.kenyon.edu/10.1038/ni1582</ref> This enables NK cells to recognize tumor and infected, especially virally infected, cells without previous stimulation, which resulted in rapid and “natural” cytotoxicity. Apart from the first discovered and well-known immunosurveillance against tumor cells<ref name = Herberman1/>,<ref name = Herberman2/>,<ref name = Wolfe/>,<ref name = Eidenschenk/>, NK cells also play important roles in containing a number of microbial and viral infections (such as: cytomegalovirus, HIV-1, herpesvirus and the malaria parasite)<ref>Cerwenka, A., & Lanier, L. L. (2001). Natural Killer Cells, Viruses and Cancer. Nature Reviews Immunology, 1(1), 41. Retrieved from link: https://doi-org.libproxy.kenyon.edu/10.1038/35095564</ref>,<ref>Lodoen, M. B., & Lanier, L. L. (2006, August). Natural killer cells as an initial defense against pathogens. Current Opinion in Immunology, 18(4), 391–398. doi: https://doi.org/10.1016/j.coi.2006.05.002</ref> as well as in early stages of pregnancy.<ref>Vivier, E., Tomasello, E., Baratin, M., Walzer, T., & Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), 503–510. Retrieved from: https://doi-org.libproxy.kenyon.edu/10.1038/ni1582</ref> | |||
<br><br> | |||
==Mechanism of NK cells’ cytotoxicity== | |||
One of NK cells’ originally-described morphological characteristics was the granules inside their cytoplasm, similar to what seen in macrophages.<ref name = Herberman1/><ref name = Herberman2/> However, later research revealed that these granules bear more similarity to those in stimulated killer T-cells than those in macrophages, and are the effector organelles of cytolysis caused by killer T-cells and NK cells.<ref>Henkart M.P., Henkart P.A. (1982) Lymphocyte Mediated Cytolysis as a Secretory Phenomenon. In: Clark W.R., Golstein P. (eds) Mechanisms of Cell-Mediated Cytotoxicity. Advances in Experimental Medicine and Biology, vol 146. Springer, Boston, MA</ref> Granules in NK cells (and also stimulated killer T cells) contain proteins responsible for signaling and facilitating cell apoptosis, the most well understood of which are perforin, granzyme, calreticulin, and glycosaminoglycan.<ref>Russell, J. H., & Ley, T. J. (2002). Lymphocyte-Mediated Cytotoxicity. Annual Review of Immunology, 20(1), 323–370. doi: 10.1146/annurev.immunol.20.100201.131730</ref> The cytolytic granules also contain Fas ligands, which facilitate the Fas-medicated cytotoxicity of NK cells coexisting with the granule-exocytosis pathway<ref>Oshimi, Y., Oda, S., Honda, Y., Nagata, S., & Miyazaki, S. (n.d.). Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. The Journal of Immunology, 157(7), 2909–2915</ref>, and granulysin but knowledge about its biochemical pathways and significance within the cytolytic granules are limited.<ref>Russell, J. H., & Ley, T. J. (2002). Lymphocyte-Mediated Cytotoxicity. Annual Review of Immunology, 20(1), 323–370. doi: 10.1146/annurev.immunol.20.100201.131730</ref> | |||
[[Image:Granule.exocytosis.and.Fas.pathway.jpg|thumb|300px|right|<b>Figure 4:</b> Granule-exocytosis and Fas-mediated pathways of NK cell-mediated cytotoxicity. Link: http://flipper.diff.org/app/items/info/4636]] | |||
<b>The Granule-exocytosis model</b><br> | |||
After NK cells form bindings of their membranal receptors with ligands on the surfaces of their neighboring cells, if the binding cells are recognized as alien, “stressed” or “infected” (target cells), cytolytic granules in NK cells are transported to the site of cell contacts and fuse with NK cells’ membrane to release the cytolytic enzymes into the space between the cells’ synapses. There, perforin creates pores to form transport channels on the target cells’ membrane and other accessory proteins chaperon granzyme into the target cells, where it signals different processes of cytolysis.<ref>Russell, J. H., & Ley, T. J. (2002). Lymphocyte-Mediated Cytotoxicity. Annual Review of Immunology, 20(1), 323–370. doi: 10.1146/annurev.immunol.20.100201.131730</ref> Exact functions of different types of granzyme, apart from the prominent, heavily studied granzyme B, are yet to be understood. | |||
<br><b>Fas-mediated apoptosis of target cells</b><br> | |||
Fas is a type of receptors on the cell’s surface that belongs to the family of tumor necrosis factor (TNF)<ref>Nagata, S., & Golstein, P. (1995). The Fas death factor. Science, 267(5203), 1449–1456. doi: 10.1126/science.7533326</ref> commonly highly expressed in cells that are enduring intercellular damages and can induce apoptosis in the Fas-presenting cells when it binds with its specific ligand (the Fas ligand or FasL) on the surface of immunosurveillance lymphocytes such as killer T cells or NK cells.<ref name = Griffith>Griffiths, G. (2002). Faculty of 1000 evaluation for Localization of Fas ligand in cytoplasmic granules of CD8 cytotoxic T lymphocytes and natural killer cells: participation of Fas ligand in granule exocytosis model of cytotoxicity. F1000 - Post-Publication Peer Review of the Biomedical Literature. doi: 10.3410/f.1008896.114957</ref> Dormant NK cells contain a significant amount of transmembranal-type FasL in their cytolytic granules on the inner surface of the outer membrane where it is stored differently from the perforin<ref name = Griffith/> and, possibly, other enzymes of the granule-exocytosis pathway. Once receptors on NK cells recognize alien, infected, or transformed (tumorous) cells, they initiate polar degranulation and release FasL into the synapses between NK cells and the target cells. The FasL binds to the Fas receptor on the target cells, activating a series of caspase enzymes within the cytoplasm of the target cells, which cleave different protein structures in the target cells.<ref>Nagata, S. (1999). Fas Ligand-Induced Apoptosis. Annual Review of Genetics, 33(1), 29–55. doi: 10.1146/annurev.genet.33.1.29</ref> There has been evidence suggestive of a DNase activated by caspase, which is a crucial process of apoptosis.<ref>Enari, M., Sakahira, H., Yokoyama, H., Okawa, K., Iwamatsu, A., & Nagata, S. (1998). A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature, 391(6662), 43–50. doi: 10.1038/34112</ref> The amount of FasL, as well as perforin, within NK cells can also be rapidly increased by the stimulation of IFN-γ<ref name = Griffith/>, a cytokine that is known for stimulant activity on NK cells. The lack of FasL on readily available on the surface of unactivated NK cells is likely to avoid killing of naïve cell types that also have amount of Fas receptor on their surfaces such as hepatocytes.<ref name = Griffith/> This Fas-mediated apoptosis pathway likely co-exists with the granule-exocytosis pathway<ref name = Griffith/> as one of the commonly seen redundant phenomena in biology. | |||
<br> <br> | |||
==“Self – non-self” recognition by NK cells/ The “missing self” hypothesis== | |||
[[Image:NK.cell.zipper.model.png|thumb|300px|left|<b>Figure 5:</b> “Zipper” module of NK cells activation. In <i>Functions of Natural Killer cells (Vivier et al. 2008)</i>. Link: https://www.semanticscholar.org/paper/Functions-of-natural-killer-cells-Vivier-Tomasello/25da9b199edde64037499489383fe4f4199460f3]] | |||
NK cells have a set of inhibitory and activating receptors on their cells’ surface that binds to specific syngeneic membranal molecules (ligands) on other cells’ membranes. A “normal” combination of these binding signals (which was established by a process of NK cell education for self-tolerance <ref>Anfossi, N., André, P., Guia, S., Falk, C. S., Roetynck, S., Stewart, C. A., … Vivier, E. (2006). Human NK Cell Education by Inhibitory Receptors for MHC Class I. Immunity, 25(2), 331–342. doi: 10.1016/j.immuni.2006.06.013</ref>) would result in NK cells remaining dormant. However, if NK cells bind to other cells that present an abnormal combination of activating and inhibitory ligands on their membranes, they are activated and release cytolytic signals into the binding cells. This mechanism allows NK cells to specifically surveil for “stressed” or “altered state” cells.<ref>Lou, Z., Jevremovic, D., Billadeau, D. D., & Leibson, P. J. (2000). A Balance between Positive and Negative Signals in Cytotoxic Lymphocytes Regulates the Polarization of Lipid Rafts during the Development of Cell-Mediated Killing. Journal of Experimental Medicine, 191(2), 347–354. doi: 10.1084/jem.191.2.347</ref>,<ref>Davis, D. M., Chiu, I., Fassett, M., Cohen, G. B., Mandelboim, O., & Strominger, J. L. (1999). The human natural killer cell immune synapse. Proceedings of the National Academy of Sciences, 96(26), 15062–15067. doi: 10.1073/pnas.96.26.15062</ref> | |||
The prominent inhibitory ligand of NK cells that were originally used to distinguish between them and the killer T cells was MHC class I molecules<ref name = Herberman1/>,<ref name = Herberman2/> When first discovered, NK cells’ cytotoxicity was said to be non-restricted by MHC.<ref name = Herberman1/>,<ref name = Herberman2/> However, it was later discovered that NK cells specifically lyse infected, tumorous or MHC-deficient cells that lack MHC class I on their surfaces <ref name = Eidenschenk/>, which make them undetectable by killer T cells. This gave rise to the “missing self” hypothesis in the 1990s.<ref>Ljunggren, H.-G., & Kärre, K. (1990). In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunology Today, 11, 237–244. doi: 10.1016/0167-5699(90)90097-s</ref> The hypothesis was later expanded to the “zipper” model of NK cell activation. which includes a range of identified inhibitory and activating receptors and their corresponding ligands on NK cells’ membrane.<ref>Vivier, E., Tomasello, E., Baratin, M., Walzer, T., & Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), 503–510. Retrieved from: https://doi-org.libproxy.kenyon.edu/10.1038/ni1582</ref> | |||
<br><b>NK cells’ coevolution with viral pathogens</b><br> | |||
A hypothesis has been proposed that NK cells evolved to fill the immunological niche created by the evolution of certain pathogens and tumor cell types to downregulate MHC class I in their host cells (the main way for infected cells to present antigens and signal their “infected” state to killer T cells) and evade cytolysis by killer T cells. Example cases can be seen in murine cytomegalovirus, human cytomegalovirus, HIV and herpes simplex virus.<ref>Lodoen, M. B., & Lanier, L. L. (2005). Viral modulation of NK cell immunity. Nature Reviews Microbiology, 3(1), 59–69. doi: 10.1038/nrmicro1066</ref> | |||
<br> <br> | |||
==NK cells in evading microbial pathogens== | |||
<br><b>Archaea</b><br> | |||
No observation of specific NK cells response to this class of pathogens has been published. | |||
[[Image:NK.cells.contact.with.E.coli.PNG|thumb|300px|right|<b>Figure 6:</b> Activated cells in close contact with E. coli. In <i>Antibacterial activity of human natural killer cells (Garcia-Penarrubia et al. 1989)</i>. Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2189196/pdf/je169199.pdf]]<b>Bacteria</b><br> | |||
NK cells antibacterial varies with the species of pathogens and the concentration of NK cells within the testing medium. It has been confirmed that NK cells has antibacterial activity in certain cases against <i>Escherichia coli</i> and <i>Samonella typhi</i> (<i>in vitro</i>)<ref>Garcia-Penarrubia, P. (1989). Antibacterial activity of human natural killer cells. Journal of Experimental Medicine, 169(1), 99–113. doi: 10.1084/jem.169.1.99</ref>, <i>Mycobacterium tuberculosis</i><ref>Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF: Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 2005, 175:4611-4617</ref>, and <i>Shigella flexneri</i> (<i>in vivo</i>)<ref>Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, Di Santo JP (2005). Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol, 175, 1735-1740</ref>. Activating receptors NKp30, NKp64 and NKG2D on NK cells’ membranes have been proven to be the main activators in the lysis of intracellularly infected mononuclear phagocytes.<ref>Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF: Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 2005, 175:4611-4617</ref> NK cells’ antibacterial activity against non-intracellular bacteria is reported to be non-phagocytic and is likely to be caused by secretion of cytolytic enzymes in NK cells’ cytolytic granules based on the granule-exocytosis model discussed above, as NK cells come in close contacts with bacterial cells, obvious bacteria ghosts surrounding active NK cells, and supernatants from NK cell samples possess strong antibacterial quality.<ref>Garcia-Penarrubia, P. (1989). Antibacterial activity of human natural killer cells. Journal of Experimental Medicine, 169(1), 99–113. doi: 10.1084/jem.169.1.99</ref> However, there is not enough research in vivo on model animals (such as mice) to draw conclusions on the mechanism of this activity outside of the <i>in vitro</i> environment. | |||
[[Image:NK.cell.and.fungal.jpg|thumb|300px|left|<b>Figure 7:</b> Interplay of NK cells and fungal pathogens. In <i>Natural Killer Cells in Antifungal Immunity (Stanislaw et al. 2017)</i>. Link: https://www.frontiersin.org/articles/10.3389/fimmu.2017.01623/full]]<b>Fungi</b><br> | |||
Researches on NK cells’ response against a number of fungi such as <i>Candida albicans, Aspergillus fumigatus</i>, and <i>Cryprococcus neoformans</i> have proven that NK cells can recognize species in this class of pathogens using their membranal activating receptors (eg: CD56, CD16, and NKp30).<ref>Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe (2013) 14:387–97. doi:10.1016/j.chom.2013.09.00</ref>,<ref>Vitenshtein A, Charpak-Amikam Y, Yamin R, Bauman Y, Isaacson B, Stein N, et al. NK cell recognition of <i>Candida glabrata</i> through binding of NKp46 and NCR1 to fungal ligands Epa1, Epa6, and Epa7. Cell Host Microbe (2016) 20:527–34. doi:10.1016/j.chom.2016.09.008</ref>,<ref>Ziegler S, Weiss E, Schmitt A-L, Schlegel J, Burgert A, Terpitz U, et al. CD56 is a pathogen recognition receptor on human natural killer cells. Sci Rep (2017) 7:6138. doi:10.1038/s41598-017-06238-4</ref>,<ref>Nabavi N, Murphy JW. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect Immun (1986) 51:556–62</ref> Recognition of these fungal pathogens is followed by both direct pathogen cell damage (through granule-exocytosis of perforin<ref>Ma LL, Wang CLC, Neely GG, Epelman S, Krensky AM, Mody CH. NK cells use perforin rather than granulysin for anticryptococcal activity. J Immunol (2004) 173:3357–65. doi:10.4049/jimmunol.173.5.3357</ref> and, possibly, granulysin<ref>Schmidt, S., Tramsen, L., & Lehrnbecher, T. (2017). Natural Killer Cells in Antifungal Immunity. Frontiers in Immunology, 8. doi: 10.3389/fimmu.2017.01623</ref>) and stimulation of other stimulator (dendritic cells) and effector cells (macrophages and neutrophils) within the immune system through cytokine secretion by NK cells.<ref>Schmidt, S., Tramsen, L., & Lehrnbecher, T. (2017). Natural Killer Cells in Antifungal Immunity. Frontiers in Immunology, 8. doi: 10.3389/fimmu.2017.01623</ref> Utilization of Fas-mediated apoptosis by NK cells against fungal pathogens has been reported in yeast.<ref>Fröhlich KU, Fussi H, Ruckenstuhl C. Yeast apoptosis – from genes to pathways. Semin Cancer Biol (2007) 17:112–21. doi:10.1016/j.semcancer.2006.11.006</ref> But the molecular mechanism of this pathway is still largely unknown, and there is contradicting information<ref>Voigt J, Hünniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, et al. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis (2014) 209:616–26. doi:10.1093/infdis/jit574</ref> about whether Fas-mediated apoptosis has significance in antifungal immunity or not. | |||
| Line 19: | Line 74: | ||
< | [[Image:NK.cell.malaria.activation.jpg|thumb|300px|right|<b>Figure 8:</b> NK cells activation by malaria infected erythrocytes. In <i>Natural killer cells and innate immunity to protozoan pathogens (Korbel et al. 2004)</i>. Link: https://www.sciencedirect.com/science/article/pii/S0020751904002115]]<b>Protozoans</b><br> | ||
< | Researches have demonstrated the important role of NK cells in defense against certain parasitic diseases, most notably, malaria (caused by the <i>Plasmodium</i> genus)<ref>Stevenson, M. M., Su, Z., Sam, H., & Mohan, K. (2001). Modulation of host responses to blood-stage malaria by interleukin-12: from therapyto adjuvant activity. Microbes and Infection, 3(1), 49–59. doi: 10.1016/s1286-4579(00)01354-x</ref>, leishmaniasis (<i>Leishmania</i> genus)<ref>Rottenberg, M., Cardoni, R.L., Andersson, R., Segura, E.L., Orn, A., 1988. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanosoma cruzi infection. Scand. J. Immunol. 28, 573–582.</ref>, trypanosomiasis (<i>Trypanosoma</i> genus)<ref>Rottenberg, M., Cardoni, R.L., Andersson, R., Segura, E.L., Orn, A., 1988. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanosoma cruzi infection. Scand. J. Immunol. 28, 573–582.</ref> and toxoplasmosis (<i>Toxoplasma</i> genus)<ref>Sher, A., Oswald, I. P., Hieny, s., & Gazzinelli, R. T. (1993). Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. The Journal of Immunology, 150, 3982–3989.</ref>. Despite the reported phenomena of NK cells’ interaction with and recognition of infect cells (such as the case of red blood cells infected with Plasmodium falciparum<ref>Artavanis-Tsakonas K, Eleme K, McQueen KL, Cheng NW, Parham P, Davis DM, Riley EM: Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol 2003, 171 :5396-5405.</ref>), NK cells mainly act as a secondary stimulator in the immune response against this class of pathogens, being stimulated by IL-12 and IL-18 (cytokines from macrophages and dendritic cells), and producing IFN-γ (another cytokine), which causes proliferation of both NK cells and phagocytic macrophages.<ref>Lodoen, M. B., & Lanier, L. L. (2006, August). Natural killer cells as an initial defense against pathogens. Current Opinion in Immunology, 18(4), 391–398. doi: https://doi.org/10.1016/j.coi.2006.05.002</ref>,<ref>Korbel, D. S., Finney, O. C., & Riley, E. M. (2004). Natural killer cells and innate immunity to protozoan pathogens. International Journal for Parasitology, 34(13-14), 1517–1528. doi: 10.1016/j.ijpara.2004.10.006</ref> There have also been evidence suggesting that NK cells’ cytotoxicity has an important role in controlling malaria infection by the strain <i>Plasmodium berghei</i> in mice.<ref>Stevenson, M. M., Su, Z., Sam, H., & Mohan, K. (2001). Modulation of host responses to blood-stage malaria by interleukin-12: from therapy to adjuvant activity. Microbes and Infection, 3(1), 49–59. doi: 10.1016/s1286-4579(00)01354-x</ref>,<ref>Solomon, J., 1986. Natural cytotoxicity for Plasmodium berghei in vitro by spleen cells from susceptible and resistant rats. Immunology 59, 277–281.</ref> Yet, no certain interpretation of this phenomenon can be produced due to insufficient knowledge. | ||
[[Image:NK.cells.and.viruses.jpg|thumb|300px|left|<b>Figure 9:</b> Activations and roles of NK cells during murine cytomegalovirus infection: (a) indirect activation, and cytolytic and interferon-secreting functions; (b) direct activation. In <i>Natural killer cells as an initial defense against pathogens (Lodoen et al. 2006)</i>. Link: https://www.sciencedirect.com/science/article/pii/S0952791506000963]]<b>Viruses</b><br> | |||
Past researches have suggested that NK cells play an important role in evading infection of certain viruses such as herpesvirus, coronavirus, HIV, ebola virus, and most prominently, cytomegalovirus.<ref>Lodoen, M. B., & Lanier, L. L. (2006, August). Natural killer cells as an initial defense against pathogens. Current Opinion in Immunology, 18(4), 391–398. doi: https://doi.org/10.1016/j.coi.2006.05.002</ref>,<ref>Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P., & Salazar-Mather, T. P. (1999). NATURAL KILLER CELLS IN ANTIVIRAL DEFENSE: Function and Regulation by Innate Cytokines. Annual Review of Immunology, 17(1), 189–220. doi: 10.1146/annurev.immunol.17.1.189</ref> The most elaborated knowledge of NK cells’ antiviral activity is against cytomegalovirus with multiple studies in NK cells’ response against both human cytomegalovirus (HCMV) and murine cytomegalovirus (MCMV) in mice. Infection of cytomegalovirus is characterized by the significant down-regulation of MHC class I on the membranes of infected cells, which helps CMV to evade detection and cytolysis by the killer T cells.<ref>Cerwenka, A., & Lanier, L. L. (2001). Natural killer cells, viruses and cancer. Nature Reviews Immunology, 1(1), 41–49. doi: 10.1038/35095564</ref> Thus, NK cells play a major role in controlling CMV infection, especially at an early stage. This has been demonstrated by multiple researches where depletion of NK cells resulted in a surge in mortality by CMV infection of the host.<ref>Lodoen, M. B., & Lanier, L. L. (2006, August). Natural killer cells as an initial defense against pathogens. Current Opinion in Immunology, 18(4), 391–398. doi: https://doi.org/10.1016/j.coi.2006.05.002</ref> Apart from their direct activation resulted from contact with infected cells and cytolytic activity against those cells, NK cells are also stimulated by cytokines and chemokines secreted by macrophages and dendritic cells to produce IFN-γ, an important interferon that inhibit replication of virus in host cells.<ref>Tay, C. H., & Welsh, R. M. (1997). distinct organ-dependent mechanism for the control of murine cytomegalovirus infection by natural killer cells. Journal of Virology, 71(1), 267–275.</ref> (Figure 9) It has also been reported that MCMV in mice induce a selective outgrowth of the Ly49H+ NK cells (Ly49H is a activating receptor on NK cells’ membranes that can bind to a ligand encoded by MCMV on the membranes of infected cells<ref>Smith, H. R. C., Heusel, J. W., Mehta, I. K., Kim, S., Dorner, B. G., Naidenko, O. V., … Yokoyama, W. M. (2002). Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proceedings of the National Academy of Sciences, 99(13), 8826–8831. doi: 10.1073/pnas.092258599</ref>), which suggests that NK cells are capable of performing a certain level of selectivity in response against pathogens.<ref>Orange, J. S., Fassett, M. S., Koopman, L. A., Boyson, J. E., & Strominger, J. L. (2002). Viral evasion of natural killer cells. Nature Immunology, 3(11), 1006–1012. doi: 10.1038/ni1102-1006</ref> It has also been suggested that IFN-γ secreted by NK cells can limit replication of mouse hepatitis virus.<ref>Trifilo, M. J., Montalto-Morrison, C., Stiles, L. N., Hurst, K. R., Hardison, J. L., Manning, J. E., … Lane, T. E. (2003). CXC Chemokine Ligand 10 Controls Viral Infection in the Central Nervous System: Evidence for a Role in Innate Immune Response through Recruitment and Activation of Natural Killer Cells. Journal of Virology, 78(2), 585–594. doi: 10.1128/jvi.78.2.585-594.2004</ref> Moreover, immunization of mice with Ebola virus-like particles showed increase NK cell antiviral activity against this virus, which significantly improved survival of the mice, and these Ebola-sensitive NK cells can be transferred to naïve mice to enhance survival.<ref>Warfield, K. L., Perkins, J. G., Swenson, D. L., Deal, E. M., Bosio, C. M., Aman, M. J., … Bavari, S. (2004). Role of Natural Killer Cells in Innate Protection against Lethal Ebola Virus Infection. The Journal of Experimental Medicine, 200(2), 169–179. doi: 10.1084/jem.20032141</ref> However, therapies specifically utilizing NK cells against hepatitis and Ebola virus needs further development before they can be tested to confirm benefits on humans. | |||
<br> <br> | <br><br> | ||
==Conclusion== | |||
Despite having been heavily researched on for the past decades, there are still many aspects of natural killer cells that are unknown, especially their cytotoxic pathways and their comprehensive functions in the immune system. Moreover, natural killer cells are promising agents to develop specific cellular therapy against cancers and possible crucial players in the onset of genetic autoimmune diseases. The newly discovered immune memory function of natural killer cells also possesses potentials in the development of antiviral serums containing natural killer cells. | |||
<br><br> | |||
==References== | ==References== | ||
<references /> | <references /> | ||
<br>Edited by [Minh Pham], student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol116/biol116_Fall_2013.html BIOL 116 Information in Living Systems], 2019, [http://www.kenyon.edu/index.xml Kenyon College]. | <br>Edited by [mailto:pham2@kenyon.edu Minh Pham], student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol116/biol116_Fall_2013.html BIOL 116 Information in Living Systems], Fall, 2019, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 04:16, 25 April 2020
Overview
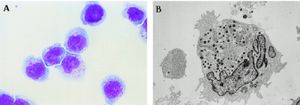
Natural killer cells (NK cells) are a type of granular cytotoxic lymphocytes that are non-adherent and non-phagocytic. NK cells were originally defined as a subset of lymphocytes that have natural cytotoxic activity against certain types of tumorous cells and endogenous type-C viruses in mice. Natural cytotoxicity refers to the fact that they can rapidly cause tumor cells’ lyses in the absence of any previous stimulation [1],[2]. They were first named in an article in 1976 [3] and later categorized as part of the innate immune system due to their morphology, origin (bone marrow), and lack of antigen-specific receptors (such as those on T and B-cells’ surfaces) and their respective genes.[4],[5]
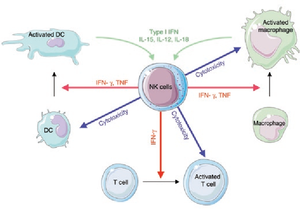
NK cells share many features with leukocytes of the innate immune system, such as: granular cytoplasm, spontaneous activity, and susceptibility to positive regulation by immune stimuli (dendritic cells’ cytokinin).[6] However, research has shown that NK cells can retain antigen-specific immunological memory [7], characteristics common to T and B-cells of the adaptive immune system, and interact with T-cells and macrophages to control immune response [8],[9], a role which is usually associated with regulatory T-cells. Furthermore, studies into the immunological reactions against cytomegalovirus in mice and human has generated evidences that certain subsets of NK cells can be activated and stimulated to multiply in response to specific pathogens.[10],[11] Thus, NK cells are now considered to be a conjunction point of both the innate and adaptive immune systems.
NK cells share a similar cytolytic mechanism of granule-exocytosis[12] with killer T cells where they utilize the two crucial cytolytic enzyme: perforin and granzyme.[13],[14],[15] Moreover, NK cells also induce apoptosis in target cells by the Fas-mediated pathway using the Fas ligands.[16] However, unlike T and B cells of the adaptive immune system, NK cells do not rely on antigen presentation by the major histocompatibility complex (MHC) class I on cells’ membranes to be activated, rather, they utilize a combination of membranal markers to distinguish between the “self” and “non-self” (or “infected self”).[17] This enables NK cells to recognize tumor and infected, especially virally infected, cells without previous stimulation, which resulted in rapid and “natural” cytotoxicity. Apart from the first discovered and well-known immunosurveillance against tumor cells[1],[2],[3],[4], NK cells also play important roles in containing a number of microbial and viral infections (such as: cytomegalovirus, HIV-1, herpesvirus and the malaria parasite)[18],[19] as well as in early stages of pregnancy.[20]
Mechanism of NK cells’ cytotoxicity
One of NK cells’ originally-described morphological characteristics was the granules inside their cytoplasm, similar to what seen in macrophages.[1][2] However, later research revealed that these granules bear more similarity to those in stimulated killer T-cells than those in macrophages, and are the effector organelles of cytolysis caused by killer T-cells and NK cells.[21] Granules in NK cells (and also stimulated killer T cells) contain proteins responsible for signaling and facilitating cell apoptosis, the most well understood of which are perforin, granzyme, calreticulin, and glycosaminoglycan.[22] The cytolytic granules also contain Fas ligands, which facilitate the Fas-medicated cytotoxicity of NK cells coexisting with the granule-exocytosis pathway[23], and granulysin but knowledge about its biochemical pathways and significance within the cytolytic granules are limited.[24]
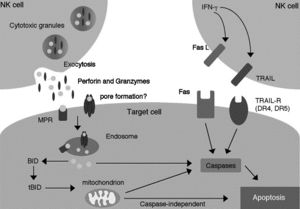
The Granule-exocytosis model
After NK cells form bindings of their membranal receptors with ligands on the surfaces of their neighboring cells, if the binding cells are recognized as alien, “stressed” or “infected” (target cells), cytolytic granules in NK cells are transported to the site of cell contacts and fuse with NK cells’ membrane to release the cytolytic enzymes into the space between the cells’ synapses. There, perforin creates pores to form transport channels on the target cells’ membrane and other accessory proteins chaperon granzyme into the target cells, where it signals different processes of cytolysis.[25] Exact functions of different types of granzyme, apart from the prominent, heavily studied granzyme B, are yet to be understood.
Fas-mediated apoptosis of target cells
Fas is a type of receptors on the cell’s surface that belongs to the family of tumor necrosis factor (TNF)[26] commonly highly expressed in cells that are enduring intercellular damages and can induce apoptosis in the Fas-presenting cells when it binds with its specific ligand (the Fas ligand or FasL) on the surface of immunosurveillance lymphocytes such as killer T cells or NK cells.[27] Dormant NK cells contain a significant amount of transmembranal-type FasL in their cytolytic granules on the inner surface of the outer membrane where it is stored differently from the perforin[27] and, possibly, other enzymes of the granule-exocytosis pathway. Once receptors on NK cells recognize alien, infected, or transformed (tumorous) cells, they initiate polar degranulation and release FasL into the synapses between NK cells and the target cells. The FasL binds to the Fas receptor on the target cells, activating a series of caspase enzymes within the cytoplasm of the target cells, which cleave different protein structures in the target cells.[28] There has been evidence suggestive of a DNase activated by caspase, which is a crucial process of apoptosis.[29] The amount of FasL, as well as perforin, within NK cells can also be rapidly increased by the stimulation of IFN-γ[27], a cytokine that is known for stimulant activity on NK cells. The lack of FasL on readily available on the surface of unactivated NK cells is likely to avoid killing of naïve cell types that also have amount of Fas receptor on their surfaces such as hepatocytes.[27] This Fas-mediated apoptosis pathway likely co-exists with the granule-exocytosis pathway[27] as one of the commonly seen redundant phenomena in biology.
“Self – non-self” recognition by NK cells/ The “missing self” hypothesis
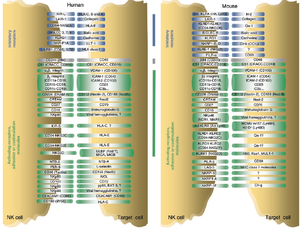
NK cells have a set of inhibitory and activating receptors on their cells’ surface that binds to specific syngeneic membranal molecules (ligands) on other cells’ membranes. A “normal” combination of these binding signals (which was established by a process of NK cell education for self-tolerance [30]) would result in NK cells remaining dormant. However, if NK cells bind to other cells that present an abnormal combination of activating and inhibitory ligands on their membranes, they are activated and release cytolytic signals into the binding cells. This mechanism allows NK cells to specifically surveil for “stressed” or “altered state” cells.[31],[32]
The prominent inhibitory ligand of NK cells that were originally used to distinguish between them and the killer T cells was MHC class I molecules[1],[2] When first discovered, NK cells’ cytotoxicity was said to be non-restricted by MHC.[1],[2] However, it was later discovered that NK cells specifically lyse infected, tumorous or MHC-deficient cells that lack MHC class I on their surfaces [4], which make them undetectable by killer T cells. This gave rise to the “missing self” hypothesis in the 1990s.[33] The hypothesis was later expanded to the “zipper” model of NK cell activation. which includes a range of identified inhibitory and activating receptors and their corresponding ligands on NK cells’ membrane.[34]
NK cells’ coevolution with viral pathogens
A hypothesis has been proposed that NK cells evolved to fill the immunological niche created by the evolution of certain pathogens and tumor cell types to downregulate MHC class I in their host cells (the main way for infected cells to present antigens and signal their “infected” state to killer T cells) and evade cytolysis by killer T cells. Example cases can be seen in murine cytomegalovirus, human cytomegalovirus, HIV and herpes simplex virus.[35]
NK cells in evading microbial pathogens
Archaea
No observation of specific NK cells response to this class of pathogens has been published.
Bacteria
NK cells antibacterial varies with the species of pathogens and the concentration of NK cells within the testing medium. It has been confirmed that NK cells has antibacterial activity in certain cases against Escherichia coli and Samonella typhi (in vitro)[36], Mycobacterium tuberculosis[37], and Shigella flexneri (in vivo)[38]. Activating receptors NKp30, NKp64 and NKG2D on NK cells’ membranes have been proven to be the main activators in the lysis of intracellularly infected mononuclear phagocytes.[39] NK cells’ antibacterial activity against non-intracellular bacteria is reported to be non-phagocytic and is likely to be caused by secretion of cytolytic enzymes in NK cells’ cytolytic granules based on the granule-exocytosis model discussed above, as NK cells come in close contacts with bacterial cells, obvious bacteria ghosts surrounding active NK cells, and supernatants from NK cell samples possess strong antibacterial quality.[40] However, there is not enough research in vivo on model animals (such as mice) to draw conclusions on the mechanism of this activity outside of the in vitro environment.
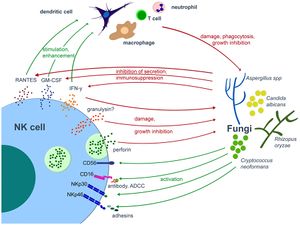
Fungi
Researches on NK cells’ response against a number of fungi such as Candida albicans, Aspergillus fumigatus, and Cryprococcus neoformans have proven that NK cells can recognize species in this class of pathogens using their membranal activating receptors (eg: CD56, CD16, and NKp30).[41],[42],[43],[44] Recognition of these fungal pathogens is followed by both direct pathogen cell damage (through granule-exocytosis of perforin[45] and, possibly, granulysin[46]) and stimulation of other stimulator (dendritic cells) and effector cells (macrophages and neutrophils) within the immune system through cytokine secretion by NK cells.[47] Utilization of Fas-mediated apoptosis by NK cells against fungal pathogens has been reported in yeast.[48] But the molecular mechanism of this pathway is still largely unknown, and there is contradicting information[49] about whether Fas-mediated apoptosis has significance in antifungal immunity or not.
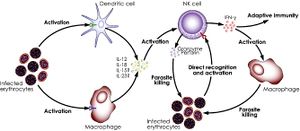
Protozoans
Researches have demonstrated the important role of NK cells in defense against certain parasitic diseases, most notably, malaria (caused by the Plasmodium genus)[50], leishmaniasis (Leishmania genus)[51], trypanosomiasis (Trypanosoma genus)[52] and toxoplasmosis (Toxoplasma genus)[53]. Despite the reported phenomena of NK cells’ interaction with and recognition of infect cells (such as the case of red blood cells infected with Plasmodium falciparum[54]), NK cells mainly act as a secondary stimulator in the immune response against this class of pathogens, being stimulated by IL-12 and IL-18 (cytokines from macrophages and dendritic cells), and producing IFN-γ (another cytokine), which causes proliferation of both NK cells and phagocytic macrophages.[55],[56] There have also been evidence suggesting that NK cells’ cytotoxicity has an important role in controlling malaria infection by the strain Plasmodium berghei in mice.[57],[58] Yet, no certain interpretation of this phenomenon can be produced due to insufficient knowledge.
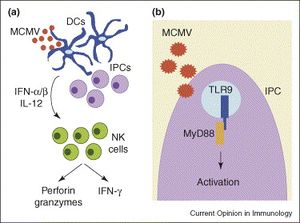
Viruses
Past researches have suggested that NK cells play an important role in evading infection of certain viruses such as herpesvirus, coronavirus, HIV, ebola virus, and most prominently, cytomegalovirus.[59],[60] The most elaborated knowledge of NK cells’ antiviral activity is against cytomegalovirus with multiple studies in NK cells’ response against both human cytomegalovirus (HCMV) and murine cytomegalovirus (MCMV) in mice. Infection of cytomegalovirus is characterized by the significant down-regulation of MHC class I on the membranes of infected cells, which helps CMV to evade detection and cytolysis by the killer T cells.[61] Thus, NK cells play a major role in controlling CMV infection, especially at an early stage. This has been demonstrated by multiple researches where depletion of NK cells resulted in a surge in mortality by CMV infection of the host.[62] Apart from their direct activation resulted from contact with infected cells and cytolytic activity against those cells, NK cells are also stimulated by cytokines and chemokines secreted by macrophages and dendritic cells to produce IFN-γ, an important interferon that inhibit replication of virus in host cells.[63] (Figure 9) It has also been reported that MCMV in mice induce a selective outgrowth of the Ly49H+ NK cells (Ly49H is a activating receptor on NK cells’ membranes that can bind to a ligand encoded by MCMV on the membranes of infected cells[64]), which suggests that NK cells are capable of performing a certain level of selectivity in response against pathogens.[65] It has also been suggested that IFN-γ secreted by NK cells can limit replication of mouse hepatitis virus.[66] Moreover, immunization of mice with Ebola virus-like particles showed increase NK cell antiviral activity against this virus, which significantly improved survival of the mice, and these Ebola-sensitive NK cells can be transferred to naïve mice to enhance survival.[67] However, therapies specifically utilizing NK cells against hepatitis and Ebola virus needs further development before they can be tested to confirm benefits on humans.
Conclusion
Despite having been heavily researched on for the past decades, there are still many aspects of natural killer cells that are unknown, especially their cytotoxic pathways and their comprehensive functions in the immune system. Moreover, natural killer cells are promising agents to develop specific cellular therapy against cancers and possible crucial players in the onset of genetic autoimmune diseases. The newly discovered immune memory function of natural killer cells also possesses potentials in the development of antiviral serums containing natural killer cells.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Herberman, R. B., Nunn, M. E., Holden, H. T. and Lavrin, D. H. (1975), Natural cytotoxic rectivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer, 16: 230-239. doi:10.1002/ijc.2910160205
- ↑ 2.0 2.1 2.2 2.3 2.4 Herberman, R. B., Nunn, M. E. and Lavrin, D. H. (1975), Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer, 16: 216-229. doi:10.1002/ijc.2910160204
- ↑ 3.0 3.1 WOLFE, S., TRACEY, D. & HENNEY, C. Induction of “natural killer” cells by BCG. Nature 262, 584–586 (1976) doi:10.1038/262584a0
- ↑ 4.0 4.1 4.2 Eidenschenk, C., Dunne, J., Jouanguy, E., Fourlinnie, C., Gineau, L., Bacq, D., … Feighery, C. (2006). A Novel Primary Immunodeficiency with Specific Natural-Killer Cell Deficiency Maps to the Centromeric Region of Chromosome 8. The American Journal of Human Genetics, 78(4), 721–727. doi: 10.1086/503269
- ↑ Trinchieri, G. Biology of natural killer cells. Adv. Immunology. Volume 47, 187-376 (1989). doi: 10.1016/S0065-2776(08)60664-1
- ↑ Trinchieri, G. Biology of natural keller cells. Adv. Immunology. Volume 47, 187-376 (1989). doi: 10.1016/S0065-2776(08)60664-1
- ↑ Sun, J., Beilke, J. & Lanier, L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009) doi:10.1038/nature07665
- ↑ Raulet, D. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol 5, 996–1002 (2004) doi:10.1038/ni1114
- ↑ Dommelen, S. L. V., Sumaria, N., Schreiber, R. D., Scalzo, A. A., Smyth, M. J., & Degli-Esposti, M. A. (2006). Perforin and Granzymes Have Distinct Roles in Defensive Immunity and Immunopathology. Immunity, 25(5), 835–848. doi: 10.1016/j.immuni.2006.09.010
- ↑ Dokun, A. O., Kim, S., Smith, H. R., Kang, H.-S. P., Chu, D. T., & Yokoyama, W. M. (2001). Specific and nonspecific NK cell activation during virus infection. Nature Immunology, 2(10), 951–956. doi: 10.1038/ni714
- ↑ Walter, L. (2011). Faculty of 1000 evaluation for Expansion of a unique CD57⁺NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. F1000 - Post-Publication Peer Review of the Biomedical Literature. doi: 10.3410/f.12631956.13874054
- ↑ Henkart M.P., Henkart P.A. (1982) Lymphocyte Mediated Cytolysis as a Secretory Phenomenon. In: Clark W.R., Golstein P. (eds) Mechanisms of Cell-Mediated Cytotoxicity. Advances in Experimental Medicine and Biology, vol 146. Springer, Boston, MA
- ↑ Dommelen, S. L. V., Sumaria, N., Schreiber, R. D., Scalzo, A. A., Smyth, M. J., & Degli-Esposti, M. A. (2006). Perforin and Granzymes Have Distinct Roles in Defensive Immunity and Immunopathology. Immunity, 25(5), 835–848. doi: 10.1016/j.immuni.2006.09.010
- ↑ Lowin, B., Beermann, F., Schmidt, A., & Tschopp, J. (1994). A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proceedings of the National Academy of Sciences, 91(24), 11571–11575. doi: 10.1073/pnas.91.24.11571
- ↑ Mahrus, S., & Craik, C. S. (2005). Selective Chemical Functional Probes of Granzymes A and B Reveal Granzyme B Is a Major Effector of Natural Killer Cell-Mediated Lysis of Target Cells. Chemistry & Biology, 12(5), 567–577. doi: 10.1016/j.chembiol.2005.03.006
- ↑ Oshimi, Y., Oda, S., Honda, Y., Nagata, S., & Miyazaki, S. (n.d.). Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. The Journal of Immunology, 157(7), 2909–2915
- ↑ Vivier, E., Tomasello, E., Baratin, M., Walzer, T., & Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), 503–510. Retrieved from: https://doi-org.libproxy.kenyon.edu/10.1038/ni1582
- ↑ Cerwenka, A., & Lanier, L. L. (2001). Natural Killer Cells, Viruses and Cancer. Nature Reviews Immunology, 1(1), 41. Retrieved from link: https://doi-org.libproxy.kenyon.edu/10.1038/35095564
- ↑ Lodoen, M. B., & Lanier, L. L. (2006, August). Natural killer cells as an initial defense against pathogens. Current Opinion in Immunology, 18(4), 391–398. doi: https://doi.org/10.1016/j.coi.2006.05.002
- ↑ Vivier, E., Tomasello, E., Baratin, M., Walzer, T., & Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), 503–510. Retrieved from: https://doi-org.libproxy.kenyon.edu/10.1038/ni1582
- ↑ Henkart M.P., Henkart P.A. (1982) Lymphocyte Mediated Cytolysis as a Secretory Phenomenon. In: Clark W.R., Golstein P. (eds) Mechanisms of Cell-Mediated Cytotoxicity. Advances in Experimental Medicine and Biology, vol 146. Springer, Boston, MA
- ↑ Russell, J. H., & Ley, T. J. (2002). Lymphocyte-Mediated Cytotoxicity. Annual Review of Immunology, 20(1), 323–370. doi: 10.1146/annurev.immunol.20.100201.131730
- ↑ Oshimi, Y., Oda, S., Honda, Y., Nagata, S., & Miyazaki, S. (n.d.). Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. The Journal of Immunology, 157(7), 2909–2915
- ↑ Russell, J. H., & Ley, T. J. (2002). Lymphocyte-Mediated Cytotoxicity. Annual Review of Immunology, 20(1), 323–370. doi: 10.1146/annurev.immunol.20.100201.131730
- ↑ Russell, J. H., & Ley, T. J. (2002). Lymphocyte-Mediated Cytotoxicity. Annual Review of Immunology, 20(1), 323–370. doi: 10.1146/annurev.immunol.20.100201.131730
- ↑ Nagata, S., & Golstein, P. (1995). The Fas death factor. Science, 267(5203), 1449–1456. doi: 10.1126/science.7533326
- ↑ 27.0 27.1 27.2 27.3 27.4 Griffiths, G. (2002). Faculty of 1000 evaluation for Localization of Fas ligand in cytoplasmic granules of CD8 cytotoxic T lymphocytes and natural killer cells: participation of Fas ligand in granule exocytosis model of cytotoxicity. F1000 - Post-Publication Peer Review of the Biomedical Literature. doi: 10.3410/f.1008896.114957
- ↑ Nagata, S. (1999). Fas Ligand-Induced Apoptosis. Annual Review of Genetics, 33(1), 29–55. doi: 10.1146/annurev.genet.33.1.29
- ↑ Enari, M., Sakahira, H., Yokoyama, H., Okawa, K., Iwamatsu, A., & Nagata, S. (1998). A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature, 391(6662), 43–50. doi: 10.1038/34112
- ↑ Anfossi, N., André, P., Guia, S., Falk, C. S., Roetynck, S., Stewart, C. A., … Vivier, E. (2006). Human NK Cell Education by Inhibitory Receptors for MHC Class I. Immunity, 25(2), 331–342. doi: 10.1016/j.immuni.2006.06.013
- ↑ Lou, Z., Jevremovic, D., Billadeau, D. D., & Leibson, P. J. (2000). A Balance between Positive and Negative Signals in Cytotoxic Lymphocytes Regulates the Polarization of Lipid Rafts during the Development of Cell-Mediated Killing. Journal of Experimental Medicine, 191(2), 347–354. doi: 10.1084/jem.191.2.347
- ↑ Davis, D. M., Chiu, I., Fassett, M., Cohen, G. B., Mandelboim, O., & Strominger, J. L. (1999). The human natural killer cell immune synapse. Proceedings of the National Academy of Sciences, 96(26), 15062–15067. doi: 10.1073/pnas.96.26.15062
- ↑ Ljunggren, H.-G., & Kärre, K. (1990). In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunology Today, 11, 237–244. doi: 10.1016/0167-5699(90)90097-s
- ↑ Vivier, E., Tomasello, E., Baratin, M., Walzer, T., & Ugolini, S. (2008). Functions of natural killer cells. Nature Immunology, 9(5), 503–510. Retrieved from: https://doi-org.libproxy.kenyon.edu/10.1038/ni1582
- ↑ Lodoen, M. B., & Lanier, L. L. (2005). Viral modulation of NK cell immunity. Nature Reviews Microbiology, 3(1), 59–69. doi: 10.1038/nrmicro1066
- ↑ Garcia-Penarrubia, P. (1989). Antibacterial activity of human natural killer cells. Journal of Experimental Medicine, 169(1), 99–113. doi: 10.1084/jem.169.1.99
- ↑ Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF: Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 2005, 175:4611-4617
- ↑ Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, Di Santo JP (2005). Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol, 175, 1735-1740
- ↑ Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF: Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 2005, 175:4611-4617
- ↑ Garcia-Penarrubia, P. (1989). Antibacterial activity of human natural killer cells. Journal of Experimental Medicine, 169(1), 99–113. doi: 10.1084/jem.169.1.99
- ↑ Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe (2013) 14:387–97. doi:10.1016/j.chom.2013.09.00
- ↑ Vitenshtein A, Charpak-Amikam Y, Yamin R, Bauman Y, Isaacson B, Stein N, et al. NK cell recognition of Candida glabrata through binding of NKp46 and NCR1 to fungal ligands Epa1, Epa6, and Epa7. Cell Host Microbe (2016) 20:527–34. doi:10.1016/j.chom.2016.09.008
- ↑ Ziegler S, Weiss E, Schmitt A-L, Schlegel J, Burgert A, Terpitz U, et al. CD56 is a pathogen recognition receptor on human natural killer cells. Sci Rep (2017) 7:6138. doi:10.1038/s41598-017-06238-4
- ↑ Nabavi N, Murphy JW. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect Immun (1986) 51:556–62
- ↑ Ma LL, Wang CLC, Neely GG, Epelman S, Krensky AM, Mody CH. NK cells use perforin rather than granulysin for anticryptococcal activity. J Immunol (2004) 173:3357–65. doi:10.4049/jimmunol.173.5.3357
- ↑ Schmidt, S., Tramsen, L., & Lehrnbecher, T. (2017). Natural Killer Cells in Antifungal Immunity. Frontiers in Immunology, 8. doi: 10.3389/fimmu.2017.01623
- ↑ Schmidt, S., Tramsen, L., & Lehrnbecher, T. (2017). Natural Killer Cells in Antifungal Immunity. Frontiers in Immunology, 8. doi: 10.3389/fimmu.2017.01623
- ↑ Fröhlich KU, Fussi H, Ruckenstuhl C. Yeast apoptosis – from genes to pathways. Semin Cancer Biol (2007) 17:112–21. doi:10.1016/j.semcancer.2006.11.006
- ↑ Voigt J, Hünniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, et al. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis (2014) 209:616–26. doi:10.1093/infdis/jit574
- ↑ Stevenson, M. M., Su, Z., Sam, H., & Mohan, K. (2001). Modulation of host responses to blood-stage malaria by interleukin-12: from therapyto adjuvant activity. Microbes and Infection, 3(1), 49–59. doi: 10.1016/s1286-4579(00)01354-x
- ↑ Rottenberg, M., Cardoni, R.L., Andersson, R., Segura, E.L., Orn, A., 1988. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanosoma cruzi infection. Scand. J. Immunol. 28, 573–582.
- ↑ Rottenberg, M., Cardoni, R.L., Andersson, R., Segura, E.L., Orn, A., 1988. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanosoma cruzi infection. Scand. J. Immunol. 28, 573–582.
- ↑ Sher, A., Oswald, I. P., Hieny, s., & Gazzinelli, R. T. (1993). Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. The Journal of Immunology, 150, 3982–3989.
- ↑ Artavanis-Tsakonas K, Eleme K, McQueen KL, Cheng NW, Parham P, Davis DM, Riley EM: Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol 2003, 171 :5396-5405.
- ↑ Lodoen, M. B., & Lanier, L. L. (2006, August). Natural killer cells as an initial defense against pathogens. Current Opinion in Immunology, 18(4), 391–398. doi: https://doi.org/10.1016/j.coi.2006.05.002
- ↑ Korbel, D. S., Finney, O. C., & Riley, E. M. (2004). Natural killer cells and innate immunity to protozoan pathogens. International Journal for Parasitology, 34(13-14), 1517–1528. doi: 10.1016/j.ijpara.2004.10.006
- ↑ Stevenson, M. M., Su, Z., Sam, H., & Mohan, K. (2001). Modulation of host responses to blood-stage malaria by interleukin-12: from therapy to adjuvant activity. Microbes and Infection, 3(1), 49–59. doi: 10.1016/s1286-4579(00)01354-x
- ↑ Solomon, J., 1986. Natural cytotoxicity for Plasmodium berghei in vitro by spleen cells from susceptible and resistant rats. Immunology 59, 277–281.
- ↑ Lodoen, M. B., & Lanier, L. L. (2006, August). Natural killer cells as an initial defense against pathogens. Current Opinion in Immunology, 18(4), 391–398. doi: https://doi.org/10.1016/j.coi.2006.05.002
- ↑ Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P., & Salazar-Mather, T. P. (1999). NATURAL KILLER CELLS IN ANTIVIRAL DEFENSE: Function and Regulation by Innate Cytokines. Annual Review of Immunology, 17(1), 189–220. doi: 10.1146/annurev.immunol.17.1.189
- ↑ Cerwenka, A., & Lanier, L. L. (2001). Natural killer cells, viruses and cancer. Nature Reviews Immunology, 1(1), 41–49. doi: 10.1038/35095564
- ↑ Lodoen, M. B., & Lanier, L. L. (2006, August). Natural killer cells as an initial defense against pathogens. Current Opinion in Immunology, 18(4), 391–398. doi: https://doi.org/10.1016/j.coi.2006.05.002
- ↑ Tay, C. H., & Welsh, R. M. (1997). distinct organ-dependent mechanism for the control of murine cytomegalovirus infection by natural killer cells. Journal of Virology, 71(1), 267–275.
- ↑ Smith, H. R. C., Heusel, J. W., Mehta, I. K., Kim, S., Dorner, B. G., Naidenko, O. V., … Yokoyama, W. M. (2002). Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proceedings of the National Academy of Sciences, 99(13), 8826–8831. doi: 10.1073/pnas.092258599
- ↑ Orange, J. S., Fassett, M. S., Koopman, L. A., Boyson, J. E., & Strominger, J. L. (2002). Viral evasion of natural killer cells. Nature Immunology, 3(11), 1006–1012. doi: 10.1038/ni1102-1006
- ↑ Trifilo, M. J., Montalto-Morrison, C., Stiles, L. N., Hurst, K. R., Hardison, J. L., Manning, J. E., … Lane, T. E. (2003). CXC Chemokine Ligand 10 Controls Viral Infection in the Central Nervous System: Evidence for a Role in Innate Immune Response through Recruitment and Activation of Natural Killer Cells. Journal of Virology, 78(2), 585–594. doi: 10.1128/jvi.78.2.585-594.2004
- ↑ Warfield, K. L., Perkins, J. G., Swenson, D. L., Deal, E. M., Bosio, C. M., Aman, M. J., … Bavari, S. (2004). Role of Natural Killer Cells in Innate Protection against Lethal Ebola Virus Infection. The Journal of Experimental Medicine, 200(2), 169–179. doi: 10.1084/jem.20032141
Edited by Minh Pham, student of Joan Slonczewski for BIOL 116 Information in Living Systems, Fall, 2019, Kenyon College.
