Nocardia cyriacigeorgica: Difference between revisions
JoshuaStorm (talk | contribs) |
|||
| (127 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
| Line 21: | Line 22: | ||
|} | |} | ||
Genus species: ''Nocardia | Genus species: ''Nocardia cyriacigeorgica'' | ||
<br>Other Names: ''Nocardia cyriacigeorgici, Nocardia cyriacigeorgica corrig''<br/> | <br>Other Names: ''Nocardia cyriacigeorgici, Nocardia cyriacigeorgica corrig''<br/> | ||
==Description and significance== | ==Description and significance== | ||
''Nocardia cyriacigeorgica'' is an | [[File:Morphology.jpg|thumb|''N. cyriacigeorgica'' morphology. Figures A-F show the growth of ''N. cyriacigeorgica'' on Columbia 5% sheep's BAP, BCYE, and Middlebrook 7H11 agar. Large colony of type VI strain ATCC 14759T (G), 100x magnification at edge of large colony (H), and 400x magnification of edge at Day 1 (I) and Day 7 (J). [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2224293/]]] | ||
''Nocardia cyriacigeorgica'' is an aerobic, gram-positive, partially acid-fast, partially branched bacteria that primarily resides in soil, characterized by dry white colonies (6). In 2001, Yassin et al. studied strain IMMIB D-1627, isolated from a patient with chronic bronchitis, and classified it as ''Nocardia cyriacigeorgici'' by utilizing a series of biochemical tests and phylogenetic evidence (7). Its genus name is derived from the work of scientist Edmond Nocard, who first isolated an aerobic actinomycete from cattle in 1888; Eppinger reported the first case of human infection, however, two years later (4). ''N. cyriacigeorgica'', until recently, was believed to be a new, emerging pathogen. But in July 2007, Pactricia S. Conville and Frank G. Witebsky at the Department of Laboratory Medicine at the National Institute of Health utilized the DNA-DNA hybridization method to conclude that ''Nocardia asteroides'' VI strains, common and well-recognized pathogen strains, belong to the ''Nocardia cyriacigeorgica'' species (2). Previous studies have found six (I-VI) existing drug pattern types of ''N. asteroides'' (12). Conville and Witebsky identified the ''Nocardia asteroides'' drug pattern VI by its reference number ATCC 14759 based on drug susceptibility patterns(2); they analyzed the 1,384 base pair regions of the 16S rRNA gene and found the reference strain of ''N. asteroides'' drug pattern type VI to be identical to the ''N. cyriacigeorgica'' strain (2). Wallace et al. discovered that strain VI showed resistance to penicillin and susceptibility to broad-spectrum cephalosporins (12). It was later placed into the subspecies ''N. asteroides'' sensu stricto along with ''N. asteroides'' I and IV, and these strains are found to be predominantly human pathogens (10). Additionally, ''N. asteroides'' VI can be also referred to as ''N. asteroides complex'', a term which also includes other similar species such as ''N. Farcinica'', ''N. Nova'', and ''N. Abscessus''(11). Fast and accurate identification of the particular ''Nocardia'' species responsible for Nocardiosis in affected individuals is thought to play a large role in the diagnosis and treatment of Nocardiosis (17). | |||
==Genome structure== | ==Genome structure== | ||
The genomic structure for ''Nocardia cyriacigeorgica'' is privately funded and not open to the public (13). ''N. cyriacigeorgica'' is 99.5% similar in HSP gene sequence as ''N. asteroides'' drug pattern VI, with only two base differences in a 373-bp region; the amino acids sequences are identical (2). Recent DNA | The genomic structure for ''Nocardia cyriacigeorgica'' is privately funded and not open to the public (13). ''N. cyriacigeorgica'' is 99.5% similar in its HSP (heat shock protein) gene sequence as ''N. asteroides'' drug pattern VI, with only two base differences in a 373-bp region; the amino acids sequences are identical (2). Recent DNA hybridization studies have shown that the two are related, and they are now believed to belong to the same species. The total genome size of ''N. asteroides'' is 7.5 Mb with a large linear plasmid of 220 kb (8). To date, only a few complete genomes of ''Nocardia'' species are available to the general public: ''Nocardia farcinica'' and ''Nocardia cyriacigeorgica'' (14).<br/> | ||
<br>''Nocardia farcinica'' has one circular chormosome and two circular plasmids. Its circular chromosome consists of 6,021,225 bp and two circular plasmids, pNF1 and pNF2 consists of 184,027bp and 87,093 bp respectively. Since ''Nocardia farcinica'' is a soil bacteria, it contains various proteins which helps it to adapt to diverse soil environment, including short-chain dehydrogenases and ABC transporters. However, it lacks PE/PPE/PGRS family proteins, | <br>''Nocardia farcinica'' has one circular chormosome and two circular plasmids. Its circular chromosome consists of 6,021,225 bp and two circular plasmids, pNF1 and pNF2 consists of 184,027bp and 87,093 bp respectively. Since ''Nocardia farcinica'' is a soil bacteria, it contains various proteins which helps it to adapt to diverse soil environment, including short-chain dehydrogenases and ABC transporters. However, it lacks PE/PPE/PGRS family proteins, proteins utilized for pathogenicity and intracellular growth, which allow it to inhabit both soil and tissue. Additionally, its ability to duplicate genes was found to enable it to be resistant to many drugs. N. farcinica possesses two β subunits for RNA polymerase, designated rpoB and rpoB2. This is the first case reported in which two rpoB genes were found on one bacterial genome (15).<br/> | ||
<br> Zoropogui et al. isolated and studied the pathogenic strain ''Nocardia cyriacigeorgica'' GUH-2 and found its complete genome to contain 6,194,645 base pairs; the strain did not contain any plasmids. Many of the genes that encode for virulence are similar to those found in ''Mycobacterium tuberculosis'' and ''Nocardia farcinica''. The genes found in the GUH-2 strain included six complete mce loci that are used for mammalian cell entry; 19 lipoproteins; hemolysin; 17 esterases, including 3 85-kDa-antigen family proteins; and 5 PE PGRS/PPE (Pro-Glu proteins that contain polymorphic GC-rich sequences, Pro-Pro-Glu) family proteins. Also found were three catalase genes and two superoxide dismutases, which were recognized to be similar to genes involved in macrophage resistance in Nocardia (19). | |||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
The Nocardia species can be distinguished phenotypically. For example, the presence of aerial hyphae | The Nocardia species can be distinguished phenotypically. For example, Nocardia are characterized by their filamentous, branched shape and the presence of aerial hyphae (11). At the early stage of development, ''N. cyriacigeorgica'' has irregular, white aerial hyphae. Later, these hyphae will start to become rod-shaped, which is typical of the Nocardia family (7). Yassin et al. discovered that ''N. cyriacigeorgica'' contained long mycolic acids with 46-54 carbon atoms (7). Studies have shown that ''N. asteroides'' strains “contain meso-diaminopimelic acid as a cell wall diamino acid and to have galactose and arabinose as characteristic whole-cell sugars” (18).''N. cyriacigeorgica'' has a slow growth rate, which makes it difficult to test for susceptibility. Individuals who are infected must be on antibiotics for an extended amount of time (17).<br/> | ||
<br> | <br>Because it is typically found in soil, ''N. cyriacigeorgica'' utilize a variety of compounds for carbon source and energy (7). Its primary sources for energy and carbon include acetate, glucose, and sucrose (7). During nitrogen assimilation, glutamate and glutamine is formed. ''N. asteroides'' utilizes the GS/GOGAT pathway to form glutamine, which contains the enzymes glutamine synthetase and glutamate synthase (9). These enzymes catalyzes glutamate from α-ketogluterate and ammonia (7). <br/> | ||
==Ecology and | ==Ecology, Pathology, and Antibiotic Susceptibility== | ||
''Nocardia cyriacigeorgica'' | ''Nocardia cyriacigeorgica'' is found primarily in soil, but can also inhabit humans and is sometimes found in water habitats (11). | ||
''Nocardia cyriacigeorgica'', or ''N. asteroides'' VI, causes most of the cases of human infection (11)and one of the | ''Nocardia cyriacigeorgica'', or ''N. asteroides'' VI, causes most of the cases of human infection (11) and is one of the species that causes Nocardiosis(17). Some diseases caused by this pathogen are, but not limited to: chronic bronchitis (10), brain abscesses (5), and most commonly pulmonary disease (14). ''N. cyriacigeorgica'' is considered to be an opportunistic infection, that is, individuals who are immunosuppressed are especially susceptible to ''Nocardia cyriacigeorgica'' (3). In a study analyzing Nocardial infections in Japan from 1992-2001, 22.4% of those infected were individuals on immunosuppressive agents, followed by SLE therapy: 3.6%, cancer: 6.6%, diabetes: 3.6%, tuberculosis: 3.3%, and AIDS 2.0%. ''Nocardia asteroides'' complex, which includes ''N. beijingensis'' and ''N. cyriacigeorgica'', was responsible for 71.4% of lung infections and 8.5% of skin infections (3)(11). Researchers suggest ''N. asteroides'' could be an etiological agent of Parkinson’s disease because of its ability to spread to the brain via the bloodstream (15). Because ''N. cyriacigeorgica'' is primarily a soil bacteria, most pulmonary infections occur through inhalation. Areas that are dry and windy are primary locations for infection because the winds enhance the relocation of the microbe (11). | ||
<br>Detection of ''Nocardia cyriacigeorgica'' is limited to gram staining and smears and cultures. Even | |||
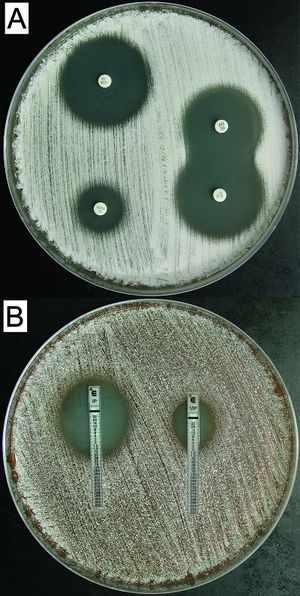
<br>Although virulence factors for ''Nocarida cyriacigeorgica'' | [[File:Susceptibility.jpg|thumb|''N. cyriacigeorgica'' susceptibility to (A) amikacin (top right), imipenem (top left, lower right), and meropenem (lower left). Minimum inhibitory concentrations (MIC) performed on imipenem (left) and meropenem (right) are show in Figure B. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2224293/]]] | ||
<br> Pulmonary nocardiosis primarily affects immoncompromised individuals and risk factors typically associated with the disease include chronic obstructive pulmonary disease (COPD), bronchiectasis, corticosteroid therapy, and cystic fibrosis; the use of corticosteroid therapies and immunosuppressant agents are the main risk factors. Pulmonary nocardiosis can be categorized as acute, subacute, or chronic. Symptoms of pulmonary nocardiosis are non-specific and include fever, dyspnea, and cough. Mortality related to nocardiosis is roughly 15% (21). | |||
<br>Detection of ''Nocardia cyriacigeorgica'' is limited to gram staining and smears and cultures. Even acid-fast stains are used primarily to confirm whether the specimen is acid-fast or not; they cannot be considered definitive tools of diagnosis, however (11).<br/> | |||
<br>Although virulence factors for ''Nocarida cyriacigeorgica'' are currently unknown, there are 6 copies of the Mce proteins found on another species in the Nocardia family: ''Nocardia farcinica''. The c-terminus of the protein Nfa34810 found in ''Nocardia farcinica'' plays an important role in invasion and adherence to host cells (15). Once ''N. cyriacigeorgica'' invades its host, it can inhibit phagosome-lysosome fusion in phagocytes, modifying its functions and allowing itself to grow within phagocytes. T-cells and macrophages have been found to be the primary immune cells emitted by the immune system in response to ''N. cyriacigeorgica'' infection (16).<br/> | |||
<br>The diagnosis and treatment of Nocardiosis, caused by the ''Nocardia asteroides'' complex can be incredibly difficult to treat. Members belonging to the complex, ''N. cyriacigeorgica'' included, although susceptible to treatments such as co-trimoxazole, show different responses to other treatment. Isolating species microscopically and phenotypically are generally time consuming and ineffective methods (17). ''N. cyriacigeorgica'' and type VI strains are typically susceptible to sulfonamide, amikacin, linezolid, imipenem, and broad-spectrum cephalosporins, while remaining resistant to clarithromycin, ciprofloxacin, and penicillins (20). Amikacin is the most often used aminoglycoside to treat nocardial infections in the United States; it is usually combined with a carbapenem, like imipenem (20). Mortality seems to be associated with the site of infection and causative species and can be as high as 50% (20). | |||
==Current Research== | ==Current Research== | ||
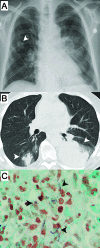
Patricia S. Conville and Frank G. Witebsky determined that Nocardia asteroides Drug Pattern Type VI are members of ''Nocardia cyriacigeorgica''. They compared drug strain ATCC 14759 to a strain of ''N. cyriacigeorgica'' | [[File:N._cyriacigeorgica.gif|thumb|''N. cyriacigeorgica'' nodules shown via posterior-anterior chest X ray (A) and computerized tomography (B). Biopsy from left lung of patient shows evidence of gram-positive, branching ''N. cyricigeorgica'' (C) [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2224293/]]] | ||
<br>Sameer Elsayed et al. reported two cases of ''Nocardia cyriageorgica'' bloodstream infection in humans; both cases | Recently, Patricia S. Conville and Frank G. Witebsky determined that ''Nocardia asteroides'' Drug Pattern Type VI are members of ''Nocardia cyriacigeorgica''. They compared drug strain ATCC 14759 to a strain of ''N. cyriacigeorgica'' by performing DNA-DNA hybridization. Later, a comparison sequence analysis of the 16S rRNA showed that there was a 99.5% similarity between the HSP gene sequence. Additionally, the amino acid sequences of the HSPs were identical. Further comparison of a region in the secA1 gene showed a 99.1% similarity between the two strains (2).<br/> | ||
<br>Additionally, Sameer Elsayed et al. reported two cases of ''Nocardia cyriageorgica'' bloodstream infection in humans; both cases illustrated the propensity of infection in immunocompromised patients. Their studies showed that the isolated strains were susceptible to trimethoprim-sulfamethoxazole (TMP-SMX), imipenenem, and amikacin (4).<br/> | |||
<br>Recent studies also suggests that one of the reasons for high mortality rates of patients with Nocardiosis could be the difficulty in identifying the strain responsible for the infection on a timely fashion. Since ''N. asteroides'' complex contains many strains and has slow growth rates, the isolation process and susceptibility process takes a long time. Furthermore, all species shows different patterns in their responses to treatment (17). This further complicates in the identification of the unknown species. | |||
<br>In January of 2008, Schlaberg, Huard, and Della-Latta described the first case of invasive ''N. cyriacigeorgica'' pulmonary infection in the United States that had been identified to the species level by utilizing 16S rRNA and hsp65 sequence analyses (20). They found that N. cyriacigeorgica is "coincident" with ''Nocardia asteroides'' complex type VI, but clearly different from the ''N. asteroides'' sensu stricto strain ATCC 19247T (20). | |||
In 2011, Riviere et al. reported a new pulmonary infection caused by ''N. cyriacigeorgica''. The researchers found that the infection was a rare, atypical infection that did not exhibit the risk factors and symptoms associated with typical Nocardial infection; the patient became infected without previously being immunocompromised or receiving corticosteroid therapies, and only presented a mild case of COPD (chronic obstructive pulmonary disease). Thus, the researchers believed there is a correlation between patients with COPD and pulmonary nocardiosis. Individuals who are affected by COPD have a higher susceptibility to fungal infection because of several possible reasons; COPD can cause changes in bronchial shape, frequent hospitalization, increased use of antibiotics and/or steroids, and comorbidity factors (ex: diabetes, alcoholism). These changes caused by COPD may increase the likelihood of ''Nocardia'' colonization and/or infection (21). | |||
==References== | ==References== | ||
| Line 79: | Line 97: | ||
(16)Beaman, B.L., and Beaman L., “Nocardia species: host-parasite relationships” <u>Clinical Microbiology Review </u>. April 1994, vol. 7(2), p. 213-264[http://cmr.asm.org/cgi/content/abstract/7/2/213 Link to Article] | (16)Beaman, B.L., and Beaman L., “Nocardia species: host-parasite relationships” <u>Clinical Microbiology Review </u>. April 1994, vol. 7(2), p. 213-264[http://cmr.asm.org/cgi/content/abstract/7/2/213 Link to Article] | ||
(17)Munoz, J., Mirelis, B., Aragon, L.M., Gutierrez, N., Sanchez, F., Espanol, M., Esparcia, O., Gurgui, Mn., Domingo, P., and Coll, P., “Clinical and microbiological features of nocardiosis 1997-2003” <u>Journal of Medical Microbiology</u>. 2007, vol. 56, p. 545-550[http://jmm.sgmjournals.org/cgi/content/abstract/56/4/545 Link to Article] | |||
(18)Hideki Yamamura, Masayuki Hayakawa, Youji Nakagawa, and Yuzuru Iimura “Characterization of Nocardia asteroides Isolates from Different Ecological Habitats on the Basis of Repetitive Extragenic Palindromic-PCR Fingerprinting” <u> applied and Environmental Microbiology</u> 2004 May; 70(5): 3149–3151 [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=404384 Link to Article] | |||
(19)Zoropogui, Anthony, Peter Pujic, et al. "Genome Sequence of the Human- and Animal-Pathogenic Strain Nocardia cyriacigeorgica GUH-2." Journal of Bacteriology. 194.8 (2012): 2098-2099. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3318486/ Link to Article] | |||
(20)Schlaberg, Robert, Richard Huard, and Phyllis Della-Latta. "Nocardia cyriacigeorgica, an emerging pathogen in the United States." Journal of Clinical Microbiology. 46.1 (2008): 265-273. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2224293/ Link to Article] | |||
(21)Reviere, F., et al. "Pulmonary nocardiosis in immunocompetent patients: can COPD be the only risk factor?" European Respiratory Review. 20.121 (2011): 210-212. [http://err.ersjournals.com/content/20/121/210.full Link to Article] | |||
Edited by Yizhao Li student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | Edited by Yizhao Li student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | ||
Latest revision as of 21:58, 4 December 2012
A Microbial Biorealm page on the genus Nocardia cyriacigeorgica
Classification
Higher order taxa
Domain: Bacteria
Phylum: Actinobacteria
Class: Actinobacteridae
Subclass: Actinomycetales
Order: Corynebacterineae
Suborder: Nocardiaceae
Family: Nocardia
Strain: Nocardia cyriacigeorgica
Species
|
NCBI: Taxonomy |
Genus species: Nocardia cyriacigeorgica
Other Names: Nocardia cyriacigeorgici, Nocardia cyriacigeorgica corrig
Description and significance

Nocardia cyriacigeorgica is an aerobic, gram-positive, partially acid-fast, partially branched bacteria that primarily resides in soil, characterized by dry white colonies (6). In 2001, Yassin et al. studied strain IMMIB D-1627, isolated from a patient with chronic bronchitis, and classified it as Nocardia cyriacigeorgici by utilizing a series of biochemical tests and phylogenetic evidence (7). Its genus name is derived from the work of scientist Edmond Nocard, who first isolated an aerobic actinomycete from cattle in 1888; Eppinger reported the first case of human infection, however, two years later (4). N. cyriacigeorgica, until recently, was believed to be a new, emerging pathogen. But in July 2007, Pactricia S. Conville and Frank G. Witebsky at the Department of Laboratory Medicine at the National Institute of Health utilized the DNA-DNA hybridization method to conclude that Nocardia asteroides VI strains, common and well-recognized pathogen strains, belong to the Nocardia cyriacigeorgica species (2). Previous studies have found six (I-VI) existing drug pattern types of N. asteroides (12). Conville and Witebsky identified the Nocardia asteroides drug pattern VI by its reference number ATCC 14759 based on drug susceptibility patterns(2); they analyzed the 1,384 base pair regions of the 16S rRNA gene and found the reference strain of N. asteroides drug pattern type VI to be identical to the N. cyriacigeorgica strain (2). Wallace et al. discovered that strain VI showed resistance to penicillin and susceptibility to broad-spectrum cephalosporins (12). It was later placed into the subspecies N. asteroides sensu stricto along with N. asteroides I and IV, and these strains are found to be predominantly human pathogens (10). Additionally, N. asteroides VI can be also referred to as N. asteroides complex, a term which also includes other similar species such as N. Farcinica, N. Nova, and N. Abscessus(11). Fast and accurate identification of the particular Nocardia species responsible for Nocardiosis in affected individuals is thought to play a large role in the diagnosis and treatment of Nocardiosis (17).
Genome structure
The genomic structure for Nocardia cyriacigeorgica is privately funded and not open to the public (13). N. cyriacigeorgica is 99.5% similar in its HSP (heat shock protein) gene sequence as N. asteroides drug pattern VI, with only two base differences in a 373-bp region; the amino acids sequences are identical (2). Recent DNA hybridization studies have shown that the two are related, and they are now believed to belong to the same species. The total genome size of N. asteroides is 7.5 Mb with a large linear plasmid of 220 kb (8). To date, only a few complete genomes of Nocardia species are available to the general public: Nocardia farcinica and Nocardia cyriacigeorgica (14).
Nocardia farcinica has one circular chormosome and two circular plasmids. Its circular chromosome consists of 6,021,225 bp and two circular plasmids, pNF1 and pNF2 consists of 184,027bp and 87,093 bp respectively. Since Nocardia farcinica is a soil bacteria, it contains various proteins which helps it to adapt to diverse soil environment, including short-chain dehydrogenases and ABC transporters. However, it lacks PE/PPE/PGRS family proteins, proteins utilized for pathogenicity and intracellular growth, which allow it to inhabit both soil and tissue. Additionally, its ability to duplicate genes was found to enable it to be resistant to many drugs. N. farcinica possesses two β subunits for RNA polymerase, designated rpoB and rpoB2. This is the first case reported in which two rpoB genes were found on one bacterial genome (15).
Zoropogui et al. isolated and studied the pathogenic strain Nocardia cyriacigeorgica GUH-2 and found its complete genome to contain 6,194,645 base pairs; the strain did not contain any plasmids. Many of the genes that encode for virulence are similar to those found in Mycobacterium tuberculosis and Nocardia farcinica. The genes found in the GUH-2 strain included six complete mce loci that are used for mammalian cell entry; 19 lipoproteins; hemolysin; 17 esterases, including 3 85-kDa-antigen family proteins; and 5 PE PGRS/PPE (Pro-Glu proteins that contain polymorphic GC-rich sequences, Pro-Pro-Glu) family proteins. Also found were three catalase genes and two superoxide dismutases, which were recognized to be similar to genes involved in macrophage resistance in Nocardia (19).
Cell structure and metabolism
The Nocardia species can be distinguished phenotypically. For example, Nocardia are characterized by their filamentous, branched shape and the presence of aerial hyphae (11). At the early stage of development, N. cyriacigeorgica has irregular, white aerial hyphae. Later, these hyphae will start to become rod-shaped, which is typical of the Nocardia family (7). Yassin et al. discovered that N. cyriacigeorgica contained long mycolic acids with 46-54 carbon atoms (7). Studies have shown that N. asteroides strains “contain meso-diaminopimelic acid as a cell wall diamino acid and to have galactose and arabinose as characteristic whole-cell sugars” (18).N. cyriacigeorgica has a slow growth rate, which makes it difficult to test for susceptibility. Individuals who are infected must be on antibiotics for an extended amount of time (17).
Because it is typically found in soil, N. cyriacigeorgica utilize a variety of compounds for carbon source and energy (7). Its primary sources for energy and carbon include acetate, glucose, and sucrose (7). During nitrogen assimilation, glutamate and glutamine is formed. N. asteroides utilizes the GS/GOGAT pathway to form glutamine, which contains the enzymes glutamine synthetase and glutamate synthase (9). These enzymes catalyzes glutamate from α-ketogluterate and ammonia (7).
Ecology, Pathology, and Antibiotic Susceptibility
Nocardia cyriacigeorgica is found primarily in soil, but can also inhabit humans and is sometimes found in water habitats (11). Nocardia cyriacigeorgica, or N. asteroides VI, causes most of the cases of human infection (11) and is one of the species that causes Nocardiosis(17). Some diseases caused by this pathogen are, but not limited to: chronic bronchitis (10), brain abscesses (5), and most commonly pulmonary disease (14). N. cyriacigeorgica is considered to be an opportunistic infection, that is, individuals who are immunosuppressed are especially susceptible to Nocardia cyriacigeorgica (3). In a study analyzing Nocardial infections in Japan from 1992-2001, 22.4% of those infected were individuals on immunosuppressive agents, followed by SLE therapy: 3.6%, cancer: 6.6%, diabetes: 3.6%, tuberculosis: 3.3%, and AIDS 2.0%. Nocardia asteroides complex, which includes N. beijingensis and N. cyriacigeorgica, was responsible for 71.4% of lung infections and 8.5% of skin infections (3)(11). Researchers suggest N. asteroides could be an etiological agent of Parkinson’s disease because of its ability to spread to the brain via the bloodstream (15). Because N. cyriacigeorgica is primarily a soil bacteria, most pulmonary infections occur through inhalation. Areas that are dry and windy are primary locations for infection because the winds enhance the relocation of the microbe (11).
Pulmonary nocardiosis primarily affects immoncompromised individuals and risk factors typically associated with the disease include chronic obstructive pulmonary disease (COPD), bronchiectasis, corticosteroid therapy, and cystic fibrosis; the use of corticosteroid therapies and immunosuppressant agents are the main risk factors. Pulmonary nocardiosis can be categorized as acute, subacute, or chronic. Symptoms of pulmonary nocardiosis are non-specific and include fever, dyspnea, and cough. Mortality related to nocardiosis is roughly 15% (21).
Detection of Nocardia cyriacigeorgica is limited to gram staining and smears and cultures. Even acid-fast stains are used primarily to confirm whether the specimen is acid-fast or not; they cannot be considered definitive tools of diagnosis, however (11).
Although virulence factors for Nocarida cyriacigeorgica are currently unknown, there are 6 copies of the Mce proteins found on another species in the Nocardia family: Nocardia farcinica. The c-terminus of the protein Nfa34810 found in Nocardia farcinica plays an important role in invasion and adherence to host cells (15). Once N. cyriacigeorgica invades its host, it can inhibit phagosome-lysosome fusion in phagocytes, modifying its functions and allowing itself to grow within phagocytes. T-cells and macrophages have been found to be the primary immune cells emitted by the immune system in response to N. cyriacigeorgica infection (16).
The diagnosis and treatment of Nocardiosis, caused by the Nocardia asteroides complex can be incredibly difficult to treat. Members belonging to the complex, N. cyriacigeorgica included, although susceptible to treatments such as co-trimoxazole, show different responses to other treatment. Isolating species microscopically and phenotypically are generally time consuming and ineffective methods (17). N. cyriacigeorgica and type VI strains are typically susceptible to sulfonamide, amikacin, linezolid, imipenem, and broad-spectrum cephalosporins, while remaining resistant to clarithromycin, ciprofloxacin, and penicillins (20). Amikacin is the most often used aminoglycoside to treat nocardial infections in the United States; it is usually combined with a carbapenem, like imipenem (20). Mortality seems to be associated with the site of infection and causative species and can be as high as 50% (20).
Current Research
Recently, Patricia S. Conville and Frank G. Witebsky determined that Nocardia asteroides Drug Pattern Type VI are members of Nocardia cyriacigeorgica. They compared drug strain ATCC 14759 to a strain of N. cyriacigeorgica by performing DNA-DNA hybridization. Later, a comparison sequence analysis of the 16S rRNA showed that there was a 99.5% similarity between the HSP gene sequence. Additionally, the amino acid sequences of the HSPs were identical. Further comparison of a region in the secA1 gene showed a 99.1% similarity between the two strains (2).
Additionally, Sameer Elsayed et al. reported two cases of Nocardia cyriageorgica bloodstream infection in humans; both cases illustrated the propensity of infection in immunocompromised patients. Their studies showed that the isolated strains were susceptible to trimethoprim-sulfamethoxazole (TMP-SMX), imipenenem, and amikacin (4).
Recent studies also suggests that one of the reasons for high mortality rates of patients with Nocardiosis could be the difficulty in identifying the strain responsible for the infection on a timely fashion. Since N. asteroides complex contains many strains and has slow growth rates, the isolation process and susceptibility process takes a long time. Furthermore, all species shows different patterns in their responses to treatment (17). This further complicates in the identification of the unknown species.
In January of 2008, Schlaberg, Huard, and Della-Latta described the first case of invasive N. cyriacigeorgica pulmonary infection in the United States that had been identified to the species level by utilizing 16S rRNA and hsp65 sequence analyses (20). They found that N. cyriacigeorgica is "coincident" with Nocardia asteroides complex type VI, but clearly different from the N. asteroides sensu stricto strain ATCC 19247T (20).
In 2011, Riviere et al. reported a new pulmonary infection caused by N. cyriacigeorgica. The researchers found that the infection was a rare, atypical infection that did not exhibit the risk factors and symptoms associated with typical Nocardial infection; the patient became infected without previously being immunocompromised or receiving corticosteroid therapies, and only presented a mild case of COPD (chronic obstructive pulmonary disease). Thus, the researchers believed there is a correlation between patients with COPD and pulmonary nocardiosis. Individuals who are affected by COPD have a higher susceptibility to fungal infection because of several possible reasons; COPD can cause changes in bronchial shape, frequent hospitalization, increased use of antibiotics and/or steroids, and comorbidity factors (ex: diabetes, alcoholism). These changes caused by COPD may increase the likelihood of Nocardia colonization and/or infection (21).
References
(1) "Nocardia Cyriacigeorgica". NCBI Taxonomy Browser. [4].
(2)Conville, Patricia S, Witebsky, Frank G., “Organisms Designated as Nocardia asteroides Drug Pattern Type VI Are Members of the Species Nocardia cyriacigeorgica” Journal of Clinical Microbiology. July, 2007. p. 2257-2259 Link to Article
(3)Kageyma, A., K. Yazawa, J. Ishikawa, K. Hotta, K. Nishimura & Y. Mikami, “Nocardial infections in Japan from 1992 to 2001, including the first report of infection by Nocardia transvalensis” European Journal of Epidemiology . 2004 19: p. 383-389Link to Article
(4)Elsayed, S., Kealey, A., Coffin, C.S., Read, R., Megran, D., & Zhang, K., “Nocardia cyriacigeorgica Septicemia” Journal of Clinical Microbiology. Jan. 2006, p. 280-282 Link to Article
(5)Barnaud, G., Deschamps C., Manceron, V., Mortier, E., Laurent, F., Bert, F., Boiron, P., Vinceneux, P., & Branger, C. “Brain Abscess Caused by Nocardia cyriacigeorgica in a Patient with Human Immunodeficiency Virus Infection" Journal of Clinical Microbiology. Sept. 2005 p. 4895-4897 Link to Article
(6)Palaniappan, C., Gunasekaran, M., “Purification and Properties of Glutamine Synthetase from Nocardia asteroides" Current Microbiology . 1995, vol. 31, p. 193-198Link to Article
(7)Yassin, A.F., Rainey, F.A., & Steiner, U., “Nocardia cyriacigeorgici sp. Nov." International Journal of Systematic and Evolutionary Microbiology . 2001, vol. 51 p. 1419-1423 Link to Article
(8)Redenbach, M., Scheel, J., & Schmidt, U., “Chromosome topology and genome size of selected actinomycetes species” Antoine van Leeuwenhoek International Journal of General and Molecular Microbiology. 2000, Vol. 78 p. 227-235 Link to Article
(9)Palaniappan, C., Gunasekaran, M., “Ammonium assimilation in Nocardia asteroides” Mycopathologia . 1993 Vol: 124, p. 69-72Link to Article
(10)Poirel, L., Laurent, F., Naas, T., Labia, R., Boiron, P., & Nordmann, P., “Molecular and Biochemical Analysis of AST-1, a Class A β-Lactamase from Nocardia asteroides Sensu Stricto” Antimicrobial Agents and Chemotheraphy. Mar. 2001 p. 878-882Link to Article
(11)Saubolle, M.A., & Sussland, D., “MINIREVIEW-Nocardiosis: Review of Clinical and Laboratory Experience” Journal of Clinical Microbiology. Oct. 2003 p. 4497-4501Link to Article
(12)Wallace, R.J. JR., Steele, L.C., Sumter, G., & Smith, J.M., “Antimicrobial susceptibility patterns of Nocardia asteroides” Antimicrobial Agents and Chemotheraphy. Dec. 1988, p. 1776-1779Link to Article
(13)Genoscope, http://www.genoscope.cns.fr/agc/mage/wwwpkgdb/MageHome/index.php?webpage=mage
(14)Ishikawa, J., Hoshino, Y., Ishino, k., Kurita, H., Chiba, K., Fujii, S., Hattori M., Yamashita, A., Mikami, Y., Yazawa, K., and Takeda, k. “Nocardia Farcinica Genome Project Page” Link to Page
(15)Ishikawa, J., Yamashita, A., Mikami, Y., Hoshino, Y., Kurita, H., Hotta, K., Shiba, T., and Hattori, M., “The Complete genomic sequence of Nocardia farcinica IFM 10152” Communicated by Satoshi Omura, Kitasato Institute, Tokyo, Japan. Aug. 2004Link to Article
(16)Beaman, B.L., and Beaman L., “Nocardia species: host-parasite relationships” Clinical Microbiology Review . April 1994, vol. 7(2), p. 213-264Link to Article
(17)Munoz, J., Mirelis, B., Aragon, L.M., Gutierrez, N., Sanchez, F., Espanol, M., Esparcia, O., Gurgui, Mn., Domingo, P., and Coll, P., “Clinical and microbiological features of nocardiosis 1997-2003” Journal of Medical Microbiology. 2007, vol. 56, p. 545-550Link to Article
(18)Hideki Yamamura, Masayuki Hayakawa, Youji Nakagawa, and Yuzuru Iimura “Characterization of Nocardia asteroides Isolates from Different Ecological Habitats on the Basis of Repetitive Extragenic Palindromic-PCR Fingerprinting” applied and Environmental Microbiology 2004 May; 70(5): 3149–3151 Link to Article
(19)Zoropogui, Anthony, Peter Pujic, et al. "Genome Sequence of the Human- and Animal-Pathogenic Strain Nocardia cyriacigeorgica GUH-2." Journal of Bacteriology. 194.8 (2012): 2098-2099. Link to Article
(20)Schlaberg, Robert, Richard Huard, and Phyllis Della-Latta. "Nocardia cyriacigeorgica, an emerging pathogen in the United States." Journal of Clinical Microbiology. 46.1 (2008): 265-273. Link to Article
(21)Reviere, F., et al. "Pulmonary nocardiosis in immunocompetent patients: can COPD be the only risk factor?" European Respiratory Review. 20.121 (2011): 210-212. Link to Article
Edited by Yizhao Li student of Rachel Larsen
