Ocular Infection by Chlamydia trachomatis: Public Health Responses to Pathology: Difference between revisions
| Line 37: | Line 37: | ||
<i>Chlamydia trachomatis</i> is a gram-negative obligate intracellular pathogen of the Chlamydiacae family. Originally thought to be a virus, because of the organism’s dependence on key metabolic intermediates (including ATP) from biosynthetic pathways of the eukaryotic host, <i>C. trachomatis</i> is now known to have a cell wall, DNA, RNA, and ribosomes and is classified as Bacteria (Becker, 1996). The group of small non-motile, cocci-shaped bacteria consists of a single genus, Chlamydia, and includes three species. <br> | <i>Chlamydia trachomatis</i> is a gram-negative obligate intracellular pathogen of the Chlamydiacae family. Originally thought to be a virus, because of the organism’s dependence on key metabolic intermediates (including ATP) from biosynthetic pathways of the eukaryotic host, <i>C. trachomatis</i> is now known to have a cell wall, DNA, RNA, and ribosomes and is classified as Bacteria (Becker, 1996). The group of small non-motile, cocci-shaped bacteria consists of a single genus, Chlamydia, and includes three species. <br> | ||
<br> | <br> | ||
<i>Chlamydia psitacci</i> is a known pathogen causing infection in birds and a number of mammalian species, including humans. The other two Chlamydial species, <i>C. trachomatis</i> and <i>C. pneumoniae</i>, are largely limited to causing infection in human hosts including ocular trachoma infections and genital-tract infections by <i>C. trachomatis</i>, and infection of the respiratory system by <i>C. pneumoniae</i> (Mabey, Solomon and Foster 2003). Multiple distinct variations based on differences in cell wall proteins, exist within the species-level classification of <i>C. trachomatis</i> (a term known as Serotypes) displaying selectivity for certain host tissue types over others (Solomon, Peeling, Foster et al. 2004). | <i>Chlamydia psitacci</i> is a known pathogen causing infection in birds and a number of mammalian species, including humans. The other two Chlamydial species, <i>C. trachomatis</i> and <i>C. pneumoniae</i>, are largely limited to causing infection in human hosts including ocular trachoma infections and genital-tract infections by <i>C. trachomatis</i>, and infection of the respiratory system by <i>C. pneumoniae</i> (Mabey, Solomon and Foster 2003). Multiple distinct variations based on differences in cell wall proteins, exist within the species-level classification of <i>C. trachomatis</i> (a term known as Serotypes) displaying selectivity for certain host tissue types over others (Solomon, Peeling, Foster et al. 2004).<br> | ||
<br> Serovars of <i>C. trachomatis</i> are defined by letters A-K as well as Ba, Da, Ia, and Ja. Among these, serovars A, B, Ba, and C are know to cause trachoma while serotypes D-K typically cause infections of the genital-tract (Mabey, Solomon and Foster 2003). The unique clinical presentation and diagnosis of an ocular disease resulting from infection by <i>C. trachomatis </i> depends on the serovar infecting the host tissues. A serovar which typically causes infection of the genital-tract may additionally infect the mucosal tissues of an infant’s eyes during passage through the mother’s infected birth canal, causing a neonatal conjunctivitis similar to the clinical presentation of trachoma (Mabey, Solomon & Foster 2003 article). <br> | |||
<br> | |||
Unable to grow or replicate outside of a eukaryotic host cell, Chlamydia have developed a unique parasitic developmental cycle shown in Figure 2, in which the organism alters between the metabolically inert elementary body (EB) and the reticulate body (RB) capable of multiplying by binary fission within a host cell (AbdelRahman and Belland 2005). In the initial stage of infection of the host cell, the spore-like metabolically inert elementary body, attaches and enters the host cell via bacterial adhesin and receptor proteins of the host cell (although the nature of the adhesin and receptor molecules involved are not yet known). The elementary body is next taken up into an intracellular membrane-bound vacuole called an inclusion.<br> | |||
<br> | |||
During the primary differentiation stage of the cycle, elementary bodies differentiate into the larger, metabolically active reticulate bodies (BD), which proceed to undergo cycles of binary fission in the middle stages of the cycle. Transcription of bacterial protein genes begins during this differentiation step with studies suggesting genes expressed during this stage are involved with nutrient acquisition as well as modification of the inclusion to prevent lysosomal fusion in which the pathogenic bacteria can be killed by the host’s immune response production of phagolysosomes. Following cell division, the elementary bodies redifferentiate into elementary bodies during a period of secondary differentiation. Genes expressed during this stage in the cycle include genes that encode outer-membrane proteins, and proteins involved in chromosome condensation. These elementary bodies are then released outside the cell to infect neighboring host cells upon lysis of the host epithelial cell (AbdelRahman and Belland 2005).<br> | |||
<br> | |||
Under conditions of stress, as imposed by host immune suppression, nutrient deprivation, and antibiotic exposure, Chlamydia species are known to undergo an altered growth cycle in which the bacteria survive in a culture-negative state during unfavorable growth conditions (Hogan and Mathews et al. 2004). Several studies have indicated evidence of reactivation of persistent chlamydial growth and infection in vivo. One such report examined the recurrence of ocular trachoma infection in adults who had been diagnosed with the infection while living in an endemic area during childhood, who developed active trachoma years later as adults while living in a non-endemic area, indicating infection due to persistent infection reactivation rather than a re-infection (Hogan and Mathews et al. 2004). The complicated biphasic developmental life cycle of <i>C. trachomatis</i>, as well as it’s ability to survive in a persistent non-culturable growth phase and reoccur in the host years later, has significant implications for consideration of public health treatment strategies for ocular trachoma with regards to mass antibiotic administration. | |||
Every point of information REQUIRES CITATION using the citation tool shown above. | Every point of information REQUIRES CITATION using the citation tool shown above. | ||
Revision as of 02:41, 27 April 2016
Introduction and Significance
By Lydia Wolf
The World Health Organization estimates ocular infection by the organism Chlamydia trachomatis to affect approximately 1.8 million people worldwide (WHO 2015). Repeated infection by the intracellular parasite, which typically begins during childhood, causes inflammation of the skin epithelium lining the inside of the eyelids called the conjunctiva. The chronic infection and inflammation of the conjunctiva causes the eyelashes of the upper lid to turn in and rub directly against the surface of the cornea. This rubbing against the cornea leads to scarring and, when left untreated by a trachiasis surgical procedure, ultimately leads to blindness…making it the leading cause of infectious blindness worldwide (Kuper, Solomon, Buchan, et al. 2003).
The pathogenic organism Chlamydia trachomatis is transmitted through direct personal contact of discharge from the mucous membranes of the eyes and nose, as well as flies that have been in contact with infected membranes, causing trachoma. The bacteria can also be transmitted to a newborn during delivery from a mother with a genital chlamydial infection causing neonatal conjunctivitis (Mabey, Solomon and Foster 2003). Ocular trachoma is especially prevalent in poor and rural developing regions affected by environmental risk factors which include crowded living conditions, and poor water sanitation facilities, as well as limited access to health care. Children and women living in these regions are a population of particular concern, as the family unit is a significant vehicle for transmission by direct contact with previously infected family members.
During the early 19th Century, trachoma (although already present in Europe before this time) became prevalent throughout Europe due to close contact between European and Egyptian soldiers during the Napoleonic Wars. Trachoma became an increasing public health concern in Europe following the Napoleonic Wars, and was a concern for the U.S. government regarding European immigration during the early 20th century who maintained strict trachoma inspections before the embarkation of immigrants in Europe (Schlosser 2016). The discovery of trachoma’s causative agent did not occur until around 1907 when the two Austrian researchers, Halberstaedter and Prowazek, discovered the cytoplasmic inclusion bodies in scrapings from infected patients by staining and light microscopy, which were then used to inoculate the eyes of orangutans showing how the trachoma infection was spread between individuals. Further research following this initial discovery began to link ocular trachoma to newborn conjunctivitis and sexually transmitted infections in the female genital epithelium (Schlosser 2016).
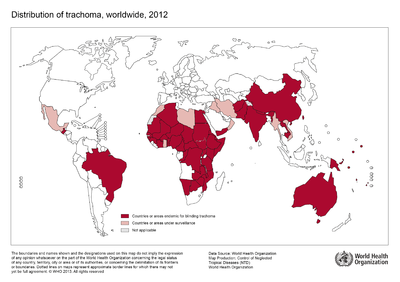
Although no longer a major health concern in Europe or the United States, trachoma remains prevalent in certain endemic regions of the developing world. The World Health Organization estimates the disease to be endemic in approximately 50 nations worldwide across Africa, Asia, and regions of the Middle East with approximately 232 million people living in these areas at risk for infection (WHO 2015). Public health campaigns are being conducted across these regions to meet goals outlined twenty years ago at the 1996 launching of the “WHO Alliance for the Global Elimination of Trachoma by the year 2020” (WHO 2015). The public health response campaigns working towards control and prevention of ocular trachoma, typically implement the WHO recommended “SAFE” strategy consisting of both environmental and medical interventions (Kuper, Solomon, Buchan, et al., 2003).
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |400px|
Placement on page: |right|
Legend/credit: World Health Organization. (2013). World Health Organization Map Production: Control of Neglected Tropical Diseases (NTD). [5].
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Sample citations: [1]
[2]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Classification, Structure, and Developmental Cycle of Chlamydia trachomatis
Chlamydia trachomatis is a gram-negative obligate intracellular pathogen of the Chlamydiacae family. Originally thought to be a virus, because of the organism’s dependence on key metabolic intermediates (including ATP) from biosynthetic pathways of the eukaryotic host, C. trachomatis is now known to have a cell wall, DNA, RNA, and ribosomes and is classified as Bacteria (Becker, 1996). The group of small non-motile, cocci-shaped bacteria consists of a single genus, Chlamydia, and includes three species.
Chlamydia psitacci is a known pathogen causing infection in birds and a number of mammalian species, including humans. The other two Chlamydial species, C. trachomatis and C. pneumoniae, are largely limited to causing infection in human hosts including ocular trachoma infections and genital-tract infections by C. trachomatis, and infection of the respiratory system by C. pneumoniae (Mabey, Solomon and Foster 2003). Multiple distinct variations based on differences in cell wall proteins, exist within the species-level classification of C. trachomatis (a term known as Serotypes) displaying selectivity for certain host tissue types over others (Solomon, Peeling, Foster et al. 2004).
Serovars of C. trachomatis are defined by letters A-K as well as Ba, Da, Ia, and Ja. Among these, serovars A, B, Ba, and C are know to cause trachoma while serotypes D-K typically cause infections of the genital-tract (Mabey, Solomon and Foster 2003). The unique clinical presentation and diagnosis of an ocular disease resulting from infection by C. trachomatis depends on the serovar infecting the host tissues. A serovar which typically causes infection of the genital-tract may additionally infect the mucosal tissues of an infant’s eyes during passage through the mother’s infected birth canal, causing a neonatal conjunctivitis similar to the clinical presentation of trachoma (Mabey, Solomon & Foster 2003 article).
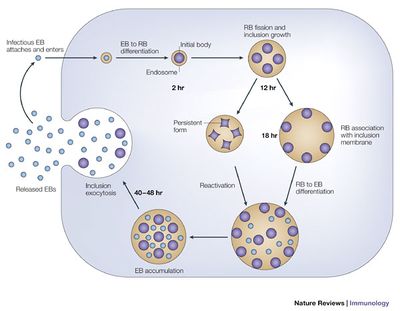
Unable to grow or replicate outside of a eukaryotic host cell, Chlamydia have developed a unique parasitic developmental cycle shown in Figure 2, in which the organism alters between the metabolically inert elementary body (EB) and the reticulate body (RB) capable of multiplying by binary fission within a host cell (AbdelRahman and Belland 2005). In the initial stage of infection of the host cell, the spore-like metabolically inert elementary body, attaches and enters the host cell via bacterial adhesin and receptor proteins of the host cell (although the nature of the adhesin and receptor molecules involved are not yet known). The elementary body is next taken up into an intracellular membrane-bound vacuole called an inclusion.
During the primary differentiation stage of the cycle, elementary bodies differentiate into the larger, metabolically active reticulate bodies (BD), which proceed to undergo cycles of binary fission in the middle stages of the cycle. Transcription of bacterial protein genes begins during this differentiation step with studies suggesting genes expressed during this stage are involved with nutrient acquisition as well as modification of the inclusion to prevent lysosomal fusion in which the pathogenic bacteria can be killed by the host’s immune response production of phagolysosomes. Following cell division, the elementary bodies redifferentiate into elementary bodies during a period of secondary differentiation. Genes expressed during this stage in the cycle include genes that encode outer-membrane proteins, and proteins involved in chromosome condensation. These elementary bodies are then released outside the cell to infect neighboring host cells upon lysis of the host epithelial cell (AbdelRahman and Belland 2005).
Under conditions of stress, as imposed by host immune suppression, nutrient deprivation, and antibiotic exposure, Chlamydia species are known to undergo an altered growth cycle in which the bacteria survive in a culture-negative state during unfavorable growth conditions (Hogan and Mathews et al. 2004). Several studies have indicated evidence of reactivation of persistent chlamydial growth and infection in vivo. One such report examined the recurrence of ocular trachoma infection in adults who had been diagnosed with the infection while living in an endemic area during childhood, who developed active trachoma years later as adults while living in a non-endemic area, indicating infection due to persistent infection reactivation rather than a re-infection (Hogan and Mathews et al. 2004). The complicated biphasic developmental life cycle of C. trachomatis, as well as it’s ability to survive in a persistent non-culturable growth phase and reoccur in the host years later, has significant implications for consideration of public health treatment strategies for ocular trachoma with regards to mass antibiotic administration.
Every point of information REQUIRES CITATION using the citation tool shown above.
Section 2
Include some current research, with at least one figure showing data.
Section 3
Include some current research, with at least one figure showing data.
Section 4

Conclusion
References
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
