Origins of a Homochiral Microbial World: Difference between revisions
No edit summary |
Slonczewski (talk | contribs) |
||
| (63 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
By: Maggie Taylor | By: Maggie Taylor | ||
==Introduction== | ==Introduction== | ||
<br>How did life begin? Where do we come from? What were the first organisms on earth? Questions like these have long puzzled even the brightest of scientists, and answers are only just beginning to surface. Historically, questions about the origins of life and evolution have evaded conversation. In societies heavily reliant on religion and faith, scientific hypotheses about the origins of life | <br>How did life begin? Where do we come from? What were the first organisms on earth? Questions like these have long puzzled even the brightest of scientists, and answers are only just beginning to surface. Historically, questions about the origins of life and evolution have evaded conversation. In societies heavily reliant on religion and faith, scientific hypotheses about the origins of life were typically ignored and deemed unsuitable to teach. In 1925, John Scopes, a biology professor, was even arrested for teachings of evolution in a classroom. Those days have faded, however, and today, society has started to use a more scientific approach to understand the origins of life on Earth. | ||
[[Image:Chemotrophs.jpg|left|thumb|250x 250 px|Chemotrophs may have been the first life forms on earth. http://www.fossilmuseum.net/Paleobiology/Precambrian-Fossils.htm]] | [[Image:Chemotrophs.jpg|left|thumb|250x 250 px|Fig. 1. Chemotrophs may have been the first life forms on earth. http://www.fossilmuseum.net/Paleobiology/Precambrian-Fossils.htm]] | ||
Microbes are the oldest life forms that have been identified today. Their simplicity, size, and metabolic diversity | |||
Microbes are the oldest life forms that have been identified today. Their simplicity, size, and metabolic diversity make them ideal candidates for life in a world without oxygen, in a world under constant attack from meteorites, and in a world where no other life forms existed. Stromatolites dating back more than 3.4 billion years depict communities of cyanobacteria in a tidal pool in the Bahamas (1, Fig. 1). Microfossils depict early microbial cells decayed by time. Biosignatures found in sedimentary rock show signs of organic molecules formed only by microbes. The question now asked is how these microbes came to be. Though microbial cells are simpler than multi-cellular, complex, eukaryotic cells, life is by no means “easy.” Enzymes, arguably the most important feature of life, are specific and highly regulated. They have evolved to be exact, even perfect. How can a world of "nothing" evolve to be perfect? | |||
[[Image:Chirality.jpg|right|thumb|250x 350 px|Enantiomers are molecules that are mirror-images of each other. | In 1848, Louis Pasteur discovered a novel way that molecules and crystals can vary without changing their molecular weight or chemical make-up. Pasteur identified two types of crystals of a solution and determined that they were the same in every way except that they were mirror images of each other. He then separated these two crystals and made solutions of each, deeming one "+" and one "-". By shining polarized light through each solution, Pasteur then demonstrated that the two solutions had equal but opposite optical activity. In other words, the direction of polarized light passing through these molecules was in opposite directions, one spinning left (L, levo), and one spinning right (D, dextro). When the two solutions were combined, now known as a racemic mixture, the solution demonstrated no optical activity. The two types of crystals, mirror images of each other, are today known as enantiomers (2, Fig. 2). | ||
[[Image:Chirality.jpg|right|thumb|250x 350 px|Fig. 2. Enantiomers are molecules that are mirror-images of each other. Today, amino acids and sugars exist in only one enantiomeric form in most biological systems on earth. This homochirality remains one of the greatest unsolved mysteries to scientists. http://www.nasa.gov/centers/jpl/news/urey-20070209.html]] | |||
Since Pasteur’s discovery of enantiomers, there has been much research in the specific orientation of atoms around organic carbon. It is generally understood that all living organisms contain sugars and amino acids of only one enantiomeric form. Thus, organisms are called “homochiral.” Homochirality is one of the most remarkable features of living organisms. In fact, it is critical to the formation of life. Enzymes act on only one enantiomer of a sugar molecule. Proteins themselves are homochiral; amino acids in living organisms are (L)-amino acids. The positioning of atoms in non-random, specific orientations, however, is actually quite difficult to do in a laboratory, especially without the aid of chiral enzymes. Kinetically, molecules prefer to isomerize in water, driving a homochiral system toward a racemic mixture(2). The prebiotic environment, if not covered by ice, would have been characterized by large waves of water that would have diluted prebiotic chiral molecules, resulting in a racemic composition of amino acids and sugars. How then did the prebiotic world select for a homochiral world? How did(L)-amino acids arise? From where did these homochiral molecules originally come? How did they survive the harsh prebiotic soup that the earth was billions of years ago? How did the world select only one enantiomer biochemically and develop an excess of that enantiomer? Answering these questions will give researchers more insight into the evolution of proteins, enzymes, and microbes on Earth. | |||
Indeed, the topic of homochirality on Earth is an active area of debate today. Three specific hypotheses incorporating all branches of the natural sciences—physics, chemistry, and biology—will provide missing links that should help scientists find more finite solutions. These hypotheses include: photolysis of one enantiomer of a racemic amino acid with circularly polarized ultraviolet light, transfer of chirality from enantioenriched amino acids to proteinogenic amino acids with a copper catalyst, and aqueous amplification of one enantiomer with preferential dissolution. | |||
<br> | <br> | ||
==Circularly Polarized Ultraviolet Light== | ==Circularly Polarized Ultraviolet Light== | ||
<br>Circularly polarized light (CPL) is an electromagnetic wave whose electric vector spirals clockwise or counterclockwise along its direction of travel. Right and left-handed CPL (R- and LCPL) appear in various places in interstellar space. For example, sunlight is rich in ultraviolet light. This light is scattered and circularly polarized at low levels, but levels are sufficient to provide a slight excess of LCPL on earth. More substantial sources of CPL are supernovas, stars at the end of their lifecycle. | <br>[[Image:CPLWAVES.gif|250× 320 px|left|thumb|Fig. 3. Circularly polarized light can be either left-handed or right-handed. http://cord.org/cm/leot/course06_mod10/Fig6.gif]]Circularly polarized light (CPL) is an electromagnetic wave whose electric vector spirals clockwise or counterclockwise along its direction of travel (Fig.3). Right and left-handed CPL (R- and LCPL) appear in various places in interstellar space. For example, sunlight is rich in ultraviolet light. This light is scattered and circularly polarized at low levels, but levels are sufficient to provide a slight excess of LCPL on earth. More substantial sources of CPL, however, are supernovas, stars at the end of their lifecycle. Upon blast, supernovas expand and pick up additional materials to form a supernova remnant (3). Synchrotron radiation from supernova remnants contains circularly polarized light. In a neutron star, this synchrotron radiation would have one chirality above a circulation plane and the opposite below it (Fig. 4). Thus, one form of circularly polarized light (for example, RCPL) might be directed into our part of the universe, while the opposite polarized light (LCPL) would be sent in the opposite direction (4).[[Image:Supernova.jpg|350× 320 px|right|thumb|Fig. 4. A supernova is a source of circular polarized light. http://www.nasa.gov/multimedia/imagegallery/image_feature_219.html]] | ||
This concept led to the hypothesis today that circular polarized light from deep within space selectively eliminated one enantiomer of a racemic mixture of amino acids on a carbonaceous chondritic meteorite, creating a slight enantiomeric excess that could eventually be transferred to Earth. In the 19th century, right and left-handed circularly polarized light, which are also mirror images of each other, were thought to be capable of stereoselectively interacting with chiral molecules. In 1897, J. H. van’t Hoff proposed the idea that optically active substances, such as enantiomers of amino acids, might be formed in nature from an interaction with R- and LCPL. It wasn’t until 1929, however, that this hypothesis was supported in a lab by Kuhn and Brown. At this time, the first successful asymmetric photolysis of a racemic substance using ultraviolet circular polarized light was accomplished and accepted by many as an agent responsible for the natural origin of optical activity (2). | |||
Today, similar studies are still undergoing to verify the assertion that circular polarized light was involved in the prebiotic foundations of optical activity on earth. Flores, et al, (1976) provided a novel approach to photolyzing a racemic amino acid (RS-leucine) with ultraviolet R- and LPCL. Using a LiF Fresnel rhomb, Flores, et al, converted linear waves of length 212.8 nm—closely approximating the desired 211 nm—to CPL (5). This novel approach had never before been achieved in a laboratory and allowed them to study the effects of R-and LCPL on a racemic mixture of leucine using gas chromatography. | |||
Unpolarized light | [[Image:RLCPL.jpg|left|thumb|650× 620 px|Table 1. Circular polarized light can be used to eliminate enantiomers in the lab. Samples treated with RCPL showed a higher composition of S-enantiomers, while treatment with LCPL showed a higher composition of R-enantiomers. Unpolarized light treatment resulted in little-to-no enantiomeric excess of the sample. http://pubs.acs.org/doi/pdf/10.1021/ja00453a018?cookieSet=1]] | ||
The results confirmed studies conducted in the 1800's. The (R)-leucine component of (RS)-leucine selectively absorbed RCPL and was degraded more extensively than the (S)-leucine component. On the other hand, the (S)-leucine component of the racemate selectively absorbed LCPL and was degraded more extensively than the (R)-leucine component. In fact, the enantiomeric excesses of samples treated with RCPL and LCPL were almost “equal and opposite” (Table 1). | |||
Unpolarized light had no selective effect on leucine enantiomer degradation. Before this publication, CPL flux in scientific studies had been insufficient at short (UV) wavelengths to have an effect on asymmetric amino acid degradation. Flores, et al, however, demonstrated the first asymmetric photolysis of amino acids using a new type of laser and even reported the second highest-reported photolysis of their racemate (5). | |||
In 1997, the discovery of meteorites with enantiomeric excesses of amino acids further revolutionized the idea of extraterrestrial origins of homochirality. Specifically, the Murchison meteorite—a carbonaceous chondrite—was discovered and dated to be more than 4.5 billion year old. [[Image:LAminoAcids.gif|350× 350 px|right|thumb|Fig. 5. Four amino acids analyzed on the Murchison Meteorite: (A) isovaline, (B) alpha -methylnorvaline, (C) alpha -amino-n-butyric acid, and (D) norvaline. Isovaline and alpha -methylnorvaline showed enantiometric excesses (6). | |||
http://www.sciencemag.org/cgi/reprint/275/5302/951.pdf]]Cronin, et al (1997) discovered that this meteorite contained a significant enantiomeric excess of two (L)-amino acids: isovaline and α-methylnorvaline (Fig. 5). | |||
Continued research in this field will support the assertion that circularly polarized light was involved in the prebiotic genesis of optical activity and the formation of homochiral molecules that could | |||
Though the excesses discovered are slight, they could be enough to indicate an extraterrestrial asymmetric influence on biochemical evolution before the origin of life. These meteorites carried homochiral molecules, and CPL from interstellar space may have played a significant role in this achievement. Because of this, the seemingly mystical concept of an "alternate universe" can now be imagined; if circular polarized light was sent in the opposite direction to the opposite side of the universe, it would influence enantiomers to form of the opposite hand of the one in which our solar system was formed. Imaginations can soar with information such as this. | |||
Continued research in this field will support the assertion that circularly polarized light was involved in the prebiotic genesis of optical activity and the formation of homochiral molecules that could later develop into biologically-active molecules on earth. | |||
<br> | <br> | ||
==Transfering Chirality to Proteinogenic Amino Acids== | |||
<br>[[Image:Synthesis.jpg|319 × 453 px|right|thumb|Fig. 6. Novel synthesis of L-alpha-Methyl-Amino Acids seen on the Murchison meteorite. http://www.dcb-server.unibe.ch/groups/reymond/education/chembiol/model_chemistry/2.pdf]]Before circular polarized light can disintegrate one enantiomer of an amino acid, the organic molecule must first be created in interstellar space. The most commonly accepted mechanism for the creation of amino acids in the universe is the Strecker reaction (7). Devised by Adolph Strecker in 1850, this reaction mechanism makes racemic α-amino acids from a carbonyl compound, ammonia, hydrogen cyanide, and water, all of which have been detected in interstellar space. Once these amino acids are formed, they can be passed to meteorite parent bodies, and as they fly by a supernova remnant, CPL emanated from the star may create an enrichment of one enantiomer (7). | |||
Indeed, the enantiomeric excess of one amino acid in meteorites demonstrates the possibility of an extraterrestrial homochiral origin. For life to arise, however, it is necessary that this homochirality be transferred to the prebiotic soup that was earth billions of years ago. One recent study proposes a novel approach to the transfer of this homochirality from an α-amino acid to biologically-relevant molecules on earth using compounds that were either present on earth or else arrived with the meteorite (7). | |||
Levine, et al (2008), demonstrate that (L)-α-methylvaline, discovered on the Murchison meteorite (Fig. 5), can transaminate phenylpyruvate to (L)-phenylalanine in a 37 % enantiomeric excess and pyruvate to (L)-alanine in up to 20 % enantiomeric excess using a copper catalyst (Fig. 7). They also demonstrate that this novel copper-catalyzed decarboxylative transamination can be extended to the synthesis of (L)-valine in the laboratory (7). | |||
[[Image:ChiralTransfer.jpg|320x 370 px|left|thumb|Fig. 7. Transamination reaction between alpha-methylvaline and a ketoacid. Enantiomer selectivity is retained with the help of a bulky copper catalyst. http://www.dcb-server.unibe.ch/groups/reymond/education/chembiol/model_chemistry/2.pdf]] | |||
Levine, et al, first provide a never-before seen synthesis of (L)-α-methylvaline with chemical means, synthesizing it in an 8 % yield from (L)-isoleucine (Fig. 6). The α-methylated amino acids were synthesized via stereospecific methylation of the corresponding cis-oxazolidinones. Methylation of amino acids is necessary for maintenance of chirality, because as a meteorite enters the earth’s atmosphere, normal amino acids—with two hydrogens on the α-carbon—will simply racemize as they rocket toward the ground. An α-methylated amino acid, however, will not racemize as it enters the earth’s atmosphere; thus, the stereocenter selected for by CPL would be maintained, and chirality would be ready to transfer to prebiotic molecules on earth. | |||
The enantiomeric | This starting methylated enantiomer, synthesized in the lab by Levine, et al, was then added in solution to a pyruvate derivative and cupric sulfate, yielding the enantiomer (L)-phenylalanine (Fig. 7). The authors obtained mechanistic insight into the enantiomeric selectivity in the copper-catalyzed reaction using highly advanced computer technology. They discovered that one potential reaction intermediate is a square planar copper (II) complex. Analysis of the van der Waals surface of this complex suggested that only one side of the α-carbon is accessible to interact with a second molecule—the other side is sterically hindered by bulky benzyl groups. Decarboxylation and selective protonation of the α-carbon from the accessible side can thus lead to a single enantiomer of (L)-phenylalanine from the homochiral(L)-α-methylisoleucine (7). | ||
Levine, et al, tested a variety of prebiotic conditions for various amounts of times and temperatures to discover optimal conditions in which their reaction would occur. They tested a temperature range of 120-160 °C, because these are the temperatures out of which biology is thought to have arisen (7). Zinc and copper were tested as potential catalysts, because they were over-abundant in the pre-biotic earth and in meteorites, but copper proved the most effective. Furthermore, the reaction experiments were conducted without solvent, as water was scarce on a hot earth, and over a long period of time. Levine, et al, discovered that the best conditions for this reaction was 160 °C for 60 minutes. Shorter reaction temperatures and times yielded a lower enantiomeric excess of phenylalanine. Indeed, these reaction conditions closely mimic conditions thought to exist on a prebiotic earth, hot and free of solvent. Levine, et al, thus provides a highly plausible transfer of homochirality from amino acids carried by parent bodies to biologically-relevant amino acids on a young earth billions of years ago (7). | |||
<br> | <br> | ||
==Aqueous Amplification of Enantiomers== | |||
<br>The transfer of homochirality from a meteorite to biological molecules on earth has been demonstrated in the laboratory. Amplifying this homochirality to a host of biological molecules, however, is another task. | |||
Racemic crystals are less soluble than homochiral crystals, because they form more stable crystals on their own thermodynamically (7). Thus, these racemic crystals are slower to dissolve in water. [[Image:Amplification.jpg|left|thumb|350x 350 px|Fig. 8. Amplification of enantiomers after preferential kinetic dissolution. http://www.dcb-server.unibe.ch/groups/reymond/education/chembiol/model_chemistry/2.pdf]]Because of this fact, in a solution of racemic and enantiomerically-enriched molecules, the racemic crystals would crystallize out of solution while the enantiomer would be enriched over time. | |||
If this solution then spread to another location, say a place downstream of a river, the enantiomeric excess would be re-located while the racemic mixture would remain behind. Evaporation of the downstream, amplified solution would then result in a sample with high enantiomeric excess of a amino acid. | |||
Because of the thermodynamic and kinetic properties of racemic crystals in solution, Levine, et al, tested to see if various proteinogenic amino acids could be amplified by preferential kinetic dissolution in prebiotic conditions (7). When the authors started with a 5 % enantiomeric excess of (L)-valine and subjected the mixture to three rounds of preferential dissolution, they found that the enantiomeric excess of the solution increased by 46 % (Fig. 8). Successive washing with ice-cold water and two more washes of preferential kinetic dissolution resulted in an additional 53 % enantiomeric enrichment. Thus, a small enantiomeric enrichment, passed from a meteorite to a proteinogenic amino acid, could be amplified to a large enrichment by successive rounds of preferential kinetic dissolution on a prebiotic earth. | |||
Amplification of homochiral molecules could have occurred in a preferential dissolution-like method if river or rainwater passed over an amino acid mixture, dissolved the enriched enantiomer more so than the racemic crystals, and then carried the enantiomer downstream to other molecules. | |||
Thus, a | |||
After this amplification of homochiral molecules, biological systems could be formed. Enantiomeric excesses would be large enough to dominate all other molecules, and racemic mixtures would | After this amplification of homochiral molecules, biological systems could be formed. Enantiomeric excesses would be large enough to dominate all other molecules, and racemic mixtures would become isolated on earth, leaving molecules, enzymes, and organisms to be created with specific homochirality (7). | ||
<br> | <br> | ||
==Implications for Future Research== | ==Implications for Future Research== | ||
<br> | <br>[[Image:1JXE.jpg|right|thumb|250x 250 px| Fig. 9. DNA polymerase is an example of an enzyme that contains only left-handed amino acids. Most proteins today contain these homochiral molecules. http://www.rcsb.org/pdb/static.do?p=explorer/viewers/jmol.jsp?structureId=1JXE]] | ||
Homochirality is necessary for the origin of life. Proteins cannot fold into bioactive configurations, such as the α-helix, if the amino acids are racemic. Enzymes cannot be efficient catalysts in early organisms if they are composed of solely racemic amino acids (Fig. 9). Enzymes made up of all D-amino acids, however, function just as well as those made up of only L-amino acids, even if the two enzymes react with the opposite stereoisomeric substrates (8). There is no biochemical reason why L-amino acids would have been favored over D-amino acids. Thus, prebiotic conditions, affected by extraterrestrial elements, must have played a significant role in the formation of specifically (L)-amino acids on earth. | |||
The vast questions that still exist after decades of searching and researching make the origins of a homochiral microbial world a hot topic in microbiology. Though scientists are making strides to find solutions, it remains to be seen whether this question will ever be answered in full. The world is full of homochiral molecules—very specific with precise roles, asymmetry, and function. These molecules must have arisen out of a universe of ‘nothing.’ Implications as to how this occurred are only just beginning to | |||
While the data supporting many of the arguments regarding the origins of a homochiral microbial world are enough to convince some people, there are many critiques that prevent this question from closing. For example, some argue that UV-CPL experiments for isovaline are not applicable, because the formation of isovaline enantiomer enrichment within the meteorite parent body would be shielded from CPL in interstellar space (9). Some also argue that amino acid symmetry breaking and amplification could have instead occurred physically, in processes such as crystallization. In brief, Kondepudi, et al (1990), reported that spontaneous chiral symmetry arises with rigorous stirring. When a solution of racemic crystals is not stirred, however, there is no rapid autocatalyzation. Asymmetric autocatalysis (the Soai reaction) with stirring, however, provided the first experimental evidence that small initial imbalances can be amplified under aqueous conditions to produce enantiomeric excess of up to 90 % (10). | |||
The vast questions that still exist after decades of searching and researching make the origins of a homochiral microbial world a hot topic in microbiology. Though scientists are making strides to find solutions, it remains to be seen whether this question will ever be answered in full. The world is full of homochiral molecules—very specific with precise roles, asymmetry, and function. These molecules must have arisen out of a universe of ‘nothing.’ Implications as to how this occurred are only just beginning to be understood. Circular polarized light, transferring chirality to proteinogenic amino acids, and the aqueous amplification of enantiomers are subjects that should be pursued in ensuing research to answer the question, 'how did a homochiral microbial world form?' | |||
<br> | <br> | ||
==References== | ==References== | ||
(1) | (1) "Precambrian Fossils." 2008. http://www.fossilmuseum.net/Paleobiology/Precambrian-Fossils.htm. | ||
(2) Bonner, William A. “The Origin and Amplification of Biomolecular Chirality.” Department of Chemistry. Stanford University. 1991. http://journals.ohiolink.edu/ejc/pdf.cgi/Bonner_William_A.pdf?issn=01696149&issue=v21i0002&article=59_toaaobc | (2) Bonner, William A. “The Origin and Amplification of Biomolecular Chirality.” Department of Chemistry. Stanford University. 1991. http://journals.ohiolink.edu/ejc/pdf.cgi/Bonner_William_A.pdf?issn=01696149&issue=v21i0002&article=59_toaaobc | ||
(3) | (3) Spitzer, Lyman. Physical Processes in the Interstellar Medium. Wiley InterScience. 2007. http://www3.interscience.wiley.com/cgi-bin/summary/117875497/SUMMARY?CRETRY=1&SRETRY=0 | ||
(4) | (4) Bailey, Jeremy, Antonio Chrysostomou, J. H. Hough, T. M. Gledhill, Alan McCall, Stuart Clark, Francois Menard, and Motohide Tamura. “Circular Polarization in Star-Formation Regions: Implications for Biomolecular Homochirality.” Science. 1998. Volume 281, p. 672-674. http://www.jstor.org/stable/2895546?seq=1 | ||
(5) | (5) Flores, Jose J., William A. Bonner, and Gail A. Massey. “Asymmetric Photolysis of (RS)-Leucine with Circularly Polarized Ultraviolet Light.” The Journal of the American Chemical Society. 1977. Volume 99, No 11. p. 3622-3624. http://pubs.acs.org/doi/pdf/10.1021/ja00453a018?cookieSet=1. | ||
(6) | (6) Cronin, John R., and Sandra Pizzarello. “Enantiomeric Excesses in Meteoritic Amino Acids.” Science. 1997. Volume 275, No 91. 951-955. http://www.sciencemag.org/cgi/reprint/275/5302/951.pdf | ||
(7) Levine, Mindy, Craig Scott Kenesky, Daniel Mazori, and Ronald Breslow. “Enantioselective Synthesis and Enantiomeric Amplification of Amino Acids under Prebiotic Conditions. Organic Letters. 2008. Volume 10, No 12. p. 2432-2436. http://www.dcb-server.unibe.ch/groups/reymond/education/chembiol/model_chemistry/2.pdf | (7) Levine, Mindy, Craig Scott Kenesky, Daniel Mazori, and Ronald Breslow. “Enantioselective Synthesis and Enantiomeric Amplification of Amino Acids under Prebiotic Conditions. Organic Letters. 2008. Volume 10, No 12. p. 2432-2436. http://www.dcb-server.unibe.ch/groups/reymond/education/chembiol/model_chemistry/2.pdf | ||
(8) " | (8) Bada, Jeffrey L. "Amino Acid Homochirality on Earth and Mars." Origins of Life and Evolution of Biospheres. 1996. Volume 26, No 3-5. 150-151. http://www.springerlink.com/content/v104j7965616l62u/fulltext.pdf. | ||
(9) Glavin, Daniel P. and Jason P. Dworkin. “Enrichment of the Amino Acid L-Isovaline by Aqueous Alteration of Cl and CM Meteorite Parent Bodies.” PNAS. 2009. | |||
( | (10) Kondepudi, Dilip K., Rebecca J. Kaufman, Nolini Singh. "Chiral Symmetry Breaking in Sodium Chlorate Crystallization." Science. 1990. Volume 250, No 4983. 975-976. http://www.jstor.org/stable/pdfplus/2878240.pdf | ||
( | (11) "NASA: Supernova Remnant Turns 400." 2004. http://www.nasa.gov/multimedia/imagegallery/image_feature_219.html | ||
( | (12) The Protein Data Bank. http://www.rcsb.org/pdb/static.do?p=explorer/viewers/jmol.jsp?structureId=1JXE. | ||
(13) "Modeule 6-10: Polarizers." http://cord.org/cm/leot/course06_mod10/Fig6.gif. | |||
Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl09.html BIOL 238 Microbiology], 2009, [http://www.kenyon.edu/index.xml Kenyon College]. | Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl09.html BIOL 238 Microbiology], 2009, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
Latest revision as of 17:57, 8 May 2012
By: Maggie Taylor
Introduction
How did life begin? Where do we come from? What were the first organisms on earth? Questions like these have long puzzled even the brightest of scientists, and answers are only just beginning to surface. Historically, questions about the origins of life and evolution have evaded conversation. In societies heavily reliant on religion and faith, scientific hypotheses about the origins of life were typically ignored and deemed unsuitable to teach. In 1925, John Scopes, a biology professor, was even arrested for teachings of evolution in a classroom. Those days have faded, however, and today, society has started to use a more scientific approach to understand the origins of life on Earth.
Microbes are the oldest life forms that have been identified today. Their simplicity, size, and metabolic diversity make them ideal candidates for life in a world without oxygen, in a world under constant attack from meteorites, and in a world where no other life forms existed. Stromatolites dating back more than 3.4 billion years depict communities of cyanobacteria in a tidal pool in the Bahamas (1, Fig. 1). Microfossils depict early microbial cells decayed by time. Biosignatures found in sedimentary rock show signs of organic molecules formed only by microbes. The question now asked is how these microbes came to be. Though microbial cells are simpler than multi-cellular, complex, eukaryotic cells, life is by no means “easy.” Enzymes, arguably the most important feature of life, are specific and highly regulated. They have evolved to be exact, even perfect. How can a world of "nothing" evolve to be perfect?
In 1848, Louis Pasteur discovered a novel way that molecules and crystals can vary without changing their molecular weight or chemical make-up. Pasteur identified two types of crystals of a solution and determined that they were the same in every way except that they were mirror images of each other. He then separated these two crystals and made solutions of each, deeming one "+" and one "-". By shining polarized light through each solution, Pasteur then demonstrated that the two solutions had equal but opposite optical activity. In other words, the direction of polarized light passing through these molecules was in opposite directions, one spinning left (L, levo), and one spinning right (D, dextro). When the two solutions were combined, now known as a racemic mixture, the solution demonstrated no optical activity. The two types of crystals, mirror images of each other, are today known as enantiomers (2, Fig. 2).
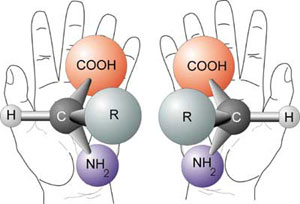
Since Pasteur’s discovery of enantiomers, there has been much research in the specific orientation of atoms around organic carbon. It is generally understood that all living organisms contain sugars and amino acids of only one enantiomeric form. Thus, organisms are called “homochiral.” Homochirality is one of the most remarkable features of living organisms. In fact, it is critical to the formation of life. Enzymes act on only one enantiomer of a sugar molecule. Proteins themselves are homochiral; amino acids in living organisms are (L)-amino acids. The positioning of atoms in non-random, specific orientations, however, is actually quite difficult to do in a laboratory, especially without the aid of chiral enzymes. Kinetically, molecules prefer to isomerize in water, driving a homochiral system toward a racemic mixture(2). The prebiotic environment, if not covered by ice, would have been characterized by large waves of water that would have diluted prebiotic chiral molecules, resulting in a racemic composition of amino acids and sugars. How then did the prebiotic world select for a homochiral world? How did(L)-amino acids arise? From where did these homochiral molecules originally come? How did they survive the harsh prebiotic soup that the earth was billions of years ago? How did the world select only one enantiomer biochemically and develop an excess of that enantiomer? Answering these questions will give researchers more insight into the evolution of proteins, enzymes, and microbes on Earth.
Indeed, the topic of homochirality on Earth is an active area of debate today. Three specific hypotheses incorporating all branches of the natural sciences—physics, chemistry, and biology—will provide missing links that should help scientists find more finite solutions. These hypotheses include: photolysis of one enantiomer of a racemic amino acid with circularly polarized ultraviolet light, transfer of chirality from enantioenriched amino acids to proteinogenic amino acids with a copper catalyst, and aqueous amplification of one enantiomer with preferential dissolution.
Circularly Polarized Ultraviolet Light
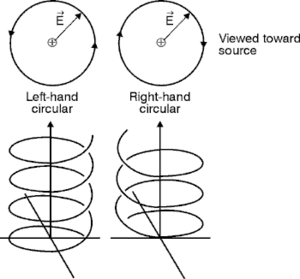
Circularly polarized light (CPL) is an electromagnetic wave whose electric vector spirals clockwise or counterclockwise along its direction of travel (Fig.3). Right and left-handed CPL (R- and LCPL) appear in various places in interstellar space. For example, sunlight is rich in ultraviolet light. This light is scattered and circularly polarized at low levels, but levels are sufficient to provide a slight excess of LCPL on earth. More substantial sources of CPL, however, are supernovas, stars at the end of their lifecycle. Upon blast, supernovas expand and pick up additional materials to form a supernova remnant (3). Synchrotron radiation from supernova remnants contains circularly polarized light. In a neutron star, this synchrotron radiation would have one chirality above a circulation plane and the opposite below it (Fig. 4). Thus, one form of circularly polarized light (for example, RCPL) might be directed into our part of the universe, while the opposite polarized light (LCPL) would be sent in the opposite direction (4).
This concept led to the hypothesis today that circular polarized light from deep within space selectively eliminated one enantiomer of a racemic mixture of amino acids on a carbonaceous chondritic meteorite, creating a slight enantiomeric excess that could eventually be transferred to Earth. In the 19th century, right and left-handed circularly polarized light, which are also mirror images of each other, were thought to be capable of stereoselectively interacting with chiral molecules. In 1897, J. H. van’t Hoff proposed the idea that optically active substances, such as enantiomers of amino acids, might be formed in nature from an interaction with R- and LCPL. It wasn’t until 1929, however, that this hypothesis was supported in a lab by Kuhn and Brown. At this time, the first successful asymmetric photolysis of a racemic substance using ultraviolet circular polarized light was accomplished and accepted by many as an agent responsible for the natural origin of optical activity (2).
Today, similar studies are still undergoing to verify the assertion that circular polarized light was involved in the prebiotic foundations of optical activity on earth. Flores, et al, (1976) provided a novel approach to photolyzing a racemic amino acid (RS-leucine) with ultraviolet R- and LPCL. Using a LiF Fresnel rhomb, Flores, et al, converted linear waves of length 212.8 nm—closely approximating the desired 211 nm—to CPL (5). This novel approach had never before been achieved in a laboratory and allowed them to study the effects of R-and LCPL on a racemic mixture of leucine using gas chromatography.

The results confirmed studies conducted in the 1800's. The (R)-leucine component of (RS)-leucine selectively absorbed RCPL and was degraded more extensively than the (S)-leucine component. On the other hand, the (S)-leucine component of the racemate selectively absorbed LCPL and was degraded more extensively than the (R)-leucine component. In fact, the enantiomeric excesses of samples treated with RCPL and LCPL were almost “equal and opposite” (Table 1).
Unpolarized light had no selective effect on leucine enantiomer degradation. Before this publication, CPL flux in scientific studies had been insufficient at short (UV) wavelengths to have an effect on asymmetric amino acid degradation. Flores, et al, however, demonstrated the first asymmetric photolysis of amino acids using a new type of laser and even reported the second highest-reported photolysis of their racemate (5).
In 1997, the discovery of meteorites with enantiomeric excesses of amino acids further revolutionized the idea of extraterrestrial origins of homochirality. Specifically, the Murchison meteorite—a carbonaceous chondrite—was discovered and dated to be more than 4.5 billion year old.
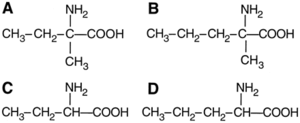
Cronin, et al (1997) discovered that this meteorite contained a significant enantiomeric excess of two (L)-amino acids: isovaline and α-methylnorvaline (Fig. 5).
Though the excesses discovered are slight, they could be enough to indicate an extraterrestrial asymmetric influence on biochemical evolution before the origin of life. These meteorites carried homochiral molecules, and CPL from interstellar space may have played a significant role in this achievement. Because of this, the seemingly mystical concept of an "alternate universe" can now be imagined; if circular polarized light was sent in the opposite direction to the opposite side of the universe, it would influence enantiomers to form of the opposite hand of the one in which our solar system was formed. Imaginations can soar with information such as this.
Continued research in this field will support the assertion that circularly polarized light was involved in the prebiotic genesis of optical activity and the formation of homochiral molecules that could later develop into biologically-active molecules on earth.
Transfering Chirality to Proteinogenic Amino Acids
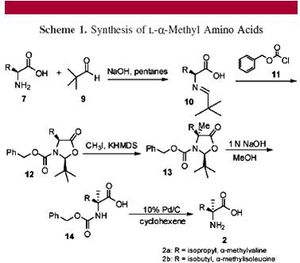
Before circular polarized light can disintegrate one enantiomer of an amino acid, the organic molecule must first be created in interstellar space. The most commonly accepted mechanism for the creation of amino acids in the universe is the Strecker reaction (7). Devised by Adolph Strecker in 1850, this reaction mechanism makes racemic α-amino acids from a carbonyl compound, ammonia, hydrogen cyanide, and water, all of which have been detected in interstellar space. Once these amino acids are formed, they can be passed to meteorite parent bodies, and as they fly by a supernova remnant, CPL emanated from the star may create an enrichment of one enantiomer (7).
Indeed, the enantiomeric excess of one amino acid in meteorites demonstrates the possibility of an extraterrestrial homochiral origin. For life to arise, however, it is necessary that this homochirality be transferred to the prebiotic soup that was earth billions of years ago. One recent study proposes a novel approach to the transfer of this homochirality from an α-amino acid to biologically-relevant molecules on earth using compounds that were either present on earth or else arrived with the meteorite (7).
Levine, et al (2008), demonstrate that (L)-α-methylvaline, discovered on the Murchison meteorite (Fig. 5), can transaminate phenylpyruvate to (L)-phenylalanine in a 37 % enantiomeric excess and pyruvate to (L)-alanine in up to 20 % enantiomeric excess using a copper catalyst (Fig. 7). They also demonstrate that this novel copper-catalyzed decarboxylative transamination can be extended to the synthesis of (L)-valine in the laboratory (7).
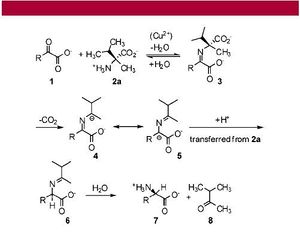
Levine, et al, first provide a never-before seen synthesis of (L)-α-methylvaline with chemical means, synthesizing it in an 8 % yield from (L)-isoleucine (Fig. 6). The α-methylated amino acids were synthesized via stereospecific methylation of the corresponding cis-oxazolidinones. Methylation of amino acids is necessary for maintenance of chirality, because as a meteorite enters the earth’s atmosphere, normal amino acids—with two hydrogens on the α-carbon—will simply racemize as they rocket toward the ground. An α-methylated amino acid, however, will not racemize as it enters the earth’s atmosphere; thus, the stereocenter selected for by CPL would be maintained, and chirality would be ready to transfer to prebiotic molecules on earth.
This starting methylated enantiomer, synthesized in the lab by Levine, et al, was then added in solution to a pyruvate derivative and cupric sulfate, yielding the enantiomer (L)-phenylalanine (Fig. 7). The authors obtained mechanistic insight into the enantiomeric selectivity in the copper-catalyzed reaction using highly advanced computer technology. They discovered that one potential reaction intermediate is a square planar copper (II) complex. Analysis of the van der Waals surface of this complex suggested that only one side of the α-carbon is accessible to interact with a second molecule—the other side is sterically hindered by bulky benzyl groups. Decarboxylation and selective protonation of the α-carbon from the accessible side can thus lead to a single enantiomer of (L)-phenylalanine from the homochiral(L)-α-methylisoleucine (7).
Levine, et al, tested a variety of prebiotic conditions for various amounts of times and temperatures to discover optimal conditions in which their reaction would occur. They tested a temperature range of 120-160 °C, because these are the temperatures out of which biology is thought to have arisen (7). Zinc and copper were tested as potential catalysts, because they were over-abundant in the pre-biotic earth and in meteorites, but copper proved the most effective. Furthermore, the reaction experiments were conducted without solvent, as water was scarce on a hot earth, and over a long period of time. Levine, et al, discovered that the best conditions for this reaction was 160 °C for 60 minutes. Shorter reaction temperatures and times yielded a lower enantiomeric excess of phenylalanine. Indeed, these reaction conditions closely mimic conditions thought to exist on a prebiotic earth, hot and free of solvent. Levine, et al, thus provides a highly plausible transfer of homochirality from amino acids carried by parent bodies to biologically-relevant amino acids on a young earth billions of years ago (7).
Aqueous Amplification of Enantiomers
The transfer of homochirality from a meteorite to biological molecules on earth has been demonstrated in the laboratory. Amplifying this homochirality to a host of biological molecules, however, is another task.
Racemic crystals are less soluble than homochiral crystals, because they form more stable crystals on their own thermodynamically (7). Thus, these racemic crystals are slower to dissolve in water.
Because of this fact, in a solution of racemic and enantiomerically-enriched molecules, the racemic crystals would crystallize out of solution while the enantiomer would be enriched over time.
If this solution then spread to another location, say a place downstream of a river, the enantiomeric excess would be re-located while the racemic mixture would remain behind. Evaporation of the downstream, amplified solution would then result in a sample with high enantiomeric excess of a amino acid.
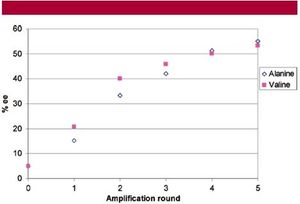
Because of the thermodynamic and kinetic properties of racemic crystals in solution, Levine, et al, tested to see if various proteinogenic amino acids could be amplified by preferential kinetic dissolution in prebiotic conditions (7). When the authors started with a 5 % enantiomeric excess of (L)-valine and subjected the mixture to three rounds of preferential dissolution, they found that the enantiomeric excess of the solution increased by 46 % (Fig. 8). Successive washing with ice-cold water and two more washes of preferential kinetic dissolution resulted in an additional 53 % enantiomeric enrichment. Thus, a small enantiomeric enrichment, passed from a meteorite to a proteinogenic amino acid, could be amplified to a large enrichment by successive rounds of preferential kinetic dissolution on a prebiotic earth.
Amplification of homochiral molecules could have occurred in a preferential dissolution-like method if river or rainwater passed over an amino acid mixture, dissolved the enriched enantiomer more so than the racemic crystals, and then carried the enantiomer downstream to other molecules.
After this amplification of homochiral molecules, biological systems could be formed. Enantiomeric excesses would be large enough to dominate all other molecules, and racemic mixtures would become isolated on earth, leaving molecules, enzymes, and organisms to be created with specific homochirality (7).
Implications for Future Research
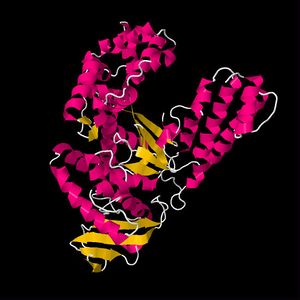
Homochirality is necessary for the origin of life. Proteins cannot fold into bioactive configurations, such as the α-helix, if the amino acids are racemic. Enzymes cannot be efficient catalysts in early organisms if they are composed of solely racemic amino acids (Fig. 9). Enzymes made up of all D-amino acids, however, function just as well as those made up of only L-amino acids, even if the two enzymes react with the opposite stereoisomeric substrates (8). There is no biochemical reason why L-amino acids would have been favored over D-amino acids. Thus, prebiotic conditions, affected by extraterrestrial elements, must have played a significant role in the formation of specifically (L)-amino acids on earth.
While the data supporting many of the arguments regarding the origins of a homochiral microbial world are enough to convince some people, there are many critiques that prevent this question from closing. For example, some argue that UV-CPL experiments for isovaline are not applicable, because the formation of isovaline enantiomer enrichment within the meteorite parent body would be shielded from CPL in interstellar space (9). Some also argue that amino acid symmetry breaking and amplification could have instead occurred physically, in processes such as crystallization. In brief, Kondepudi, et al (1990), reported that spontaneous chiral symmetry arises with rigorous stirring. When a solution of racemic crystals is not stirred, however, there is no rapid autocatalyzation. Asymmetric autocatalysis (the Soai reaction) with stirring, however, provided the first experimental evidence that small initial imbalances can be amplified under aqueous conditions to produce enantiomeric excess of up to 90 % (10).
The vast questions that still exist after decades of searching and researching make the origins of a homochiral microbial world a hot topic in microbiology. Though scientists are making strides to find solutions, it remains to be seen whether this question will ever be answered in full. The world is full of homochiral molecules—very specific with precise roles, asymmetry, and function. These molecules must have arisen out of a universe of ‘nothing.’ Implications as to how this occurred are only just beginning to be understood. Circular polarized light, transferring chirality to proteinogenic amino acids, and the aqueous amplification of enantiomers are subjects that should be pursued in ensuing research to answer the question, 'how did a homochiral microbial world form?'
References
(1) "Precambrian Fossils." 2008. http://www.fossilmuseum.net/Paleobiology/Precambrian-Fossils.htm.
(2) Bonner, William A. “The Origin and Amplification of Biomolecular Chirality.” Department of Chemistry. Stanford University. 1991. http://journals.ohiolink.edu/ejc/pdf.cgi/Bonner_William_A.pdf?issn=01696149&issue=v21i0002&article=59_toaaobc
(3) Spitzer, Lyman. Physical Processes in the Interstellar Medium. Wiley InterScience. 2007. http://www3.interscience.wiley.com/cgi-bin/summary/117875497/SUMMARY?CRETRY=1&SRETRY=0
(4) Bailey, Jeremy, Antonio Chrysostomou, J. H. Hough, T. M. Gledhill, Alan McCall, Stuart Clark, Francois Menard, and Motohide Tamura. “Circular Polarization in Star-Formation Regions: Implications for Biomolecular Homochirality.” Science. 1998. Volume 281, p. 672-674. http://www.jstor.org/stable/2895546?seq=1
(5) Flores, Jose J., William A. Bonner, and Gail A. Massey. “Asymmetric Photolysis of (RS)-Leucine with Circularly Polarized Ultraviolet Light.” The Journal of the American Chemical Society. 1977. Volume 99, No 11. p. 3622-3624. http://pubs.acs.org/doi/pdf/10.1021/ja00453a018?cookieSet=1.
(6) Cronin, John R., and Sandra Pizzarello. “Enantiomeric Excesses in Meteoritic Amino Acids.” Science. 1997. Volume 275, No 91. 951-955. http://www.sciencemag.org/cgi/reprint/275/5302/951.pdf
(7) Levine, Mindy, Craig Scott Kenesky, Daniel Mazori, and Ronald Breslow. “Enantioselective Synthesis and Enantiomeric Amplification of Amino Acids under Prebiotic Conditions. Organic Letters. 2008. Volume 10, No 12. p. 2432-2436. http://www.dcb-server.unibe.ch/groups/reymond/education/chembiol/model_chemistry/2.pdf
(8) Bada, Jeffrey L. "Amino Acid Homochirality on Earth and Mars." Origins of Life and Evolution of Biospheres. 1996. Volume 26, No 3-5. 150-151. http://www.springerlink.com/content/v104j7965616l62u/fulltext.pdf.
(9) Glavin, Daniel P. and Jason P. Dworkin. “Enrichment of the Amino Acid L-Isovaline by Aqueous Alteration of Cl and CM Meteorite Parent Bodies.” PNAS. 2009.
(10) Kondepudi, Dilip K., Rebecca J. Kaufman, Nolini Singh. "Chiral Symmetry Breaking in Sodium Chlorate Crystallization." Science. 1990. Volume 250, No 4983. 975-976. http://www.jstor.org/stable/pdfplus/2878240.pdf
(11) "NASA: Supernova Remnant Turns 400." 2004. http://www.nasa.gov/multimedia/imagegallery/image_feature_219.html
(12) The Protein Data Bank. http://www.rcsb.org/pdb/static.do?p=explorer/viewers/jmol.jsp?structureId=1JXE.
(13) "Modeule 6-10: Polarizers." http://cord.org/cm/leot/course06_mod10/Fig6.gif.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
