Pedomicrobium manganicum: Difference between revisions
| Line 38: | Line 38: | ||
'''Biogeochemical Significance''': ''Pedomicrobium manganicum'' is known to oxidize manganese[5]. This species is also known to create iron oxide and gold deposits[1][5]. | '''Biogeochemical Significance''': ''Pedomicrobium manganicum'' is known to oxidize manganese[5]. This species is also known to create iron oxide and gold deposits[1][5]. | ||
'''Contributions to Environment''': Within bioreactors of waste water and drinking water systems, this bacteria can be used to oxidize and clean out what can be called "dirty water," an excess of manganese in the water creates a murky look. Removal of manganese to reduce the murkiness of water is not the only role this bacterium plays in the environment. It is used in water supply systems to remove manganese, which at high concentrations ( 14 mg/L) is a neurotoxin [5, 9]. This bacteria can also be used as a bioremediation tool in cleaning radioactive wastes. It can remove uranium and radium from Uranium Mill Tailing Remedial Action sites (UMTRA) [5]. Since it can withstand the extreme effects of radiation, it can be used to ionize the radiation. | '''Contributions to Environment''': Within bioreactors of waste water and drinking water systems, this bacteria can be used to oxidize and clean out what can be called "dirty water," an excess of manganese in the water creates a murky look. Removal of manganese to reduce the murkiness of water is not the only role this bacterium plays in the environment. A current public health concern is that high concentrations of manganese in water affects about 8.7 million Americans [5]. To help with this issue, It is used in water supply systems to remove manganese, which at high concentrations ( 14 mg/L) is a neurotoxin [5, 9]. This bacteria can also be used as a bioremediation tool in cleaning radioactive wastes. It can remove uranium and radium from Uranium Mill Tailing Remedial Action sites (UMTRA) [5]. Since it can withstand the extreme effects of radiation, it can be used to ionize the radiation. | ||
<br> | <br> | ||
Revision as of 16:23, 4 May 2015
Classification
- Domain: Bacteria
- Phylum: Proteobacteria
- Class: Alpha Proteobacteria
- Order: Rhizobiales
- Family: Hyphomicrobiaceae
- Genus : Pedomicrobium
Species
|
NCBI: Taxonomy |
Pedomicrobium manganicum
Description and Significance
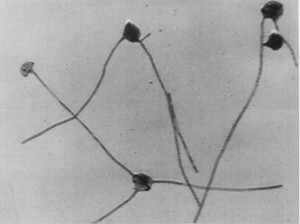
Pedomicrobium manganicum are hyphae budding bacterium. The structure of the bacterium is spherical with up to five hyphae per cell [2]. The formation of these hyphae prevent the progeny from being formed near metallic deposits [3]. As a terrestrial extremophile, it inhabits particularly harsh environments such as desert rock surfaces where it is susceptible to large variations in temperature as well as UV radiation. This microbe is most commonly found on desert rock surfaces, but it has also be found in soils, water systems, and various aquatic systems as biofilms [5]. Pedomicrobium manganicum can be used for many bioremediation processes such as removing manganese from water purification systems to the removal of uranium and radium from Uranium Mill Tailing Remedial Action sites [5].
Genome Structure
The genome of Pedomicrobium manganicum has a size of approximately 5Mb [4] and has a G/C content of 66% [5.] The rich G/C content can be attributed to two underlying reasons. Studies have shown that a higher G/C content correlates to optimal growth temperature [13]. As an extremophile living in desert environments, Pedomicrobium manganicum has a high G/C content which would be more stable at higher temperatures. Another explanation for the rich G/C content would be that higher G/C content correlates to a longer length in coding regions [12]. The number of chromosomes and weather they are circular or linear is unknown [5]. The manganese oxidation operon has been sequenced and has a Genbank Accession number of AM049177 [5].
Cell Structure, Metabolism and Life Cycle
Pedomicrobium manganicum do not rely on binary fission as their way of division. They produce prosthecas, which are simple hyphae that bud, and then form daughter cells [10]. Since this microbe dwells in extreme conditions such as desert rocks, this microbe must be able to survive in severely dry conditions. In order to do so, Pedomicrobium manganicum maintains cellular hydration by going into an anhydrobiotic state [5]. Anhydrobiosis is the process in which the cell is in an almost completely desiccated state that stabilizes its membranes and other cellular structures, preventing otherwise lethal damage caused by environmental extremes present. It acquires energy through the process of oxidation, in which electrons are stripped from manganese or iron. The products of the metabolism from Pedomicrobium manganicum include oxidized manganese and iron, as well as gold deposits [1,5].
Ecology and Pathogenesis
Biogeochemical Significance: Pedomicrobium manganicum is known to oxidize manganese[5]. This species is also known to create iron oxide and gold deposits[1][5].
Contributions to Environment: Within bioreactors of waste water and drinking water systems, this bacteria can be used to oxidize and clean out what can be called "dirty water," an excess of manganese in the water creates a murky look. Removal of manganese to reduce the murkiness of water is not the only role this bacterium plays in the environment. A current public health concern is that high concentrations of manganese in water affects about 8.7 million Americans [5]. To help with this issue, It is used in water supply systems to remove manganese, which at high concentrations ( 14 mg/L) is a neurotoxin [5, 9]. This bacteria can also be used as a bioremediation tool in cleaning radioactive wastes. It can remove uranium and radium from Uranium Mill Tailing Remedial Action sites (UMTRA) [5]. Since it can withstand the extreme effects of radiation, it can be used to ionize the radiation.
Evolutionary Impact : Rather than in vitro study of biofilm in clinical situations, in situ biofilm research can be performed on the biofilm found on desert rock surfaces. This could give insight on the evolutionary processes behind the formation of biofilms [5].
References
- Watterson, John R. "Preliminary Evidence for the Involvement of Budding Bacteria in the Origin of Alaskan Placer Gold." Geology 20.April (1992): 315-18. GeoScienceWorld. Web. 20 Apr. 2015.
- Gebers,R. "Enrichment, Isolation, and Emended Description of Pedomicrobium Ferrugineum Aristovskaya and Pedomicrobium Manganicum Aristovskaya." International Journal of Systematic Bacteriology 31.3 (1981): 302-16. IJSEM. International Journal of Systematic Bacteriology. Web. 21 Apr. 2015.
- Moore, R.L., The Biology of Hyphomicrobium and other Prosthecate, Budding Bacteria. Ann. Rev. Microbiol., 1981. 35: p. 567-594
- Koelbel-Boelke, J.G., R., Hirsch, P., Genome size determinations for 33 strains of budding bacteria. . Int. J. Syst. Bacteriol. 1985. 35: p. 270-273.
- Mackenzie, Ronald C. The Genome of the Desert Rock-surface Dwelling Bacterium Pedomicrobium Manganicum. N.p., n.d. Web.
- Nielsen FH. Ultratrace minerals. In: Shils M, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins; 1999:283-303.
- Keen CL, Zidenberg-Cherr S. Manganese. In: Ziegler EE, Filer LJ, eds. Present Knowledge in Nutrition. 7th ed. Washington D.C.: ILSI Press; 1996:334-343.
- Kawamura R. Intoxication by manganese in well water. Kisasato Archives of Experimental Medicine. 1941;18:145-169.
- Keen CL, Ensunsa JL, Watson MH, et al. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999;20(2-3):213-223.
- Hirsch, P., Budding bacteria. Annu. Rev. Microbiol., 1974. 28: p. 391-444.
- Santin, Aldo. "City to Ease the Manganese." Winnipeg Free Press. N.p., 31 Jan. 2014. Web. 26 Apr. 2015.
- Oliver, JL and Marin, A. A relationshiip between GC content and coding-sequence length. J Mol Evol 1996 Sep;43(3)216-23
- Galtier, N and Lobry JR. Relationships between genomic G+C content, RNA secondary structures, and optimal growth temperature in prokaryotes. J Mol Evol 1997 Jun;44(6)632-6
Author
Page authored by Shuaib Mirani and Derrick Martin, students of Prof. Jay Lennon at IndianaUniversity.