Plasmodium Falciparum Control Strategies
Plasmodium Falciparum Control Strategies
International effors to address Plasmodium falciparum, a protozoan parasite that causes malaria, have been ongoing since the 1940s and 1950s12. Over half of the world's population is at risk for getting infected with Plasmodium falciparum, thereby contracting malaria1.
Malaria is a deadly disease and is estimated to be endemic in over 100 different countries1. It is also the cause of approximately one million deaths every year1.
Introduction
Background
The Plasmodium falciparum parasite lives in blood, and is of the genus Plasmodium. There are over 200 different Plasmodium species, but only 11 known types actually infect humans. Of the different species, Plasmodium falciparum is the most dangerous to humans, because it has very high mortality rates2.

The main vector for the spread of the Plasmodium falciparum parasite is mosquitoes2. There are many types of mosquitoes, but only a subset suck blood; of those, only some species are malaria transmitters. The mosquito species Anopheles gambiae (pictured at right) is one of the most efficient malaria vectors known because it transmits Plasmodium falciparum2.
Spread and Life Cycle
The Plasmodium falciparum parasite population requires both human hosts and mosquito vectors in order to perpetuate. The life cycle of P. falciparum, depicted at the left, occurs as follows:10
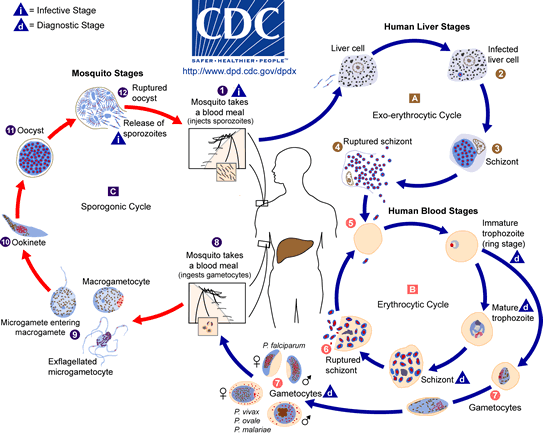
Mosquitoes acquire the P. falciparum parasite at first by ingesting blood from an infected human. The parasite reproduces when it enters the mosquito gut, and spreads to its salivary glands. When a mosquito punctures the skin of a human, the Plasmodium falciparum parasite enters the bloodstream by being released from the salivary glands of the mosquito. Upon entering the bloodstream, the P. falciparum is in sporozoite form. The sporozoites make their way to the liver, where they infect liver cells and multiply. They are then released into the bloodstream as merozoites, where they can infect red blood cells and reproduce asexually. As the cycle continues, infection spreads from human to human.
Effect on Mosquitoes
Plasmodium falciparum can cause behavioral changes in the mosquitoes that carry it.4 A mosquito that has acquired the P. falciparum parasite, is more inclined to feed off of a greater number of individuals in a night, and suck more blood in a single feeding than a mosquito without the parasite. This phenomenon increases the ability of Plasmodium falciparum to spread quickly through mosquitoes.
Control Strategies
There are no effective vaccines for malaria, since infected individuals don't obtain complete immunity to the infection. Any long-lasting immunity to malaria usually requires repeated exposure to the parasite2. The difficulty in creating any effective immunization is due to the parasite's ability to change the antigens it presents, which are the targets for protective antibodies2. The P. falciparum genome actually encodes fewer enzymes and transporters than other free-living eukaryotic microbes, but it encodes a high proportion of genes dedicated to evading immune responses and host-parasite interactions 3. There are drugs which have been used to effectively treat malaria after an infection has already occured. These include chloroquine, primaquine, and artemisinin-based combination therapy (ACT)2.
Since is not possible to completely immunize at-risk individuals, the focus of malaria prevention is on reducing or controlling the spread of the disease. Research on the microbial interactions of Plasmodium falciparum and its vector, Anopheles gambiae, reveals possible approaches for malaria control, including use of the natural mosquito immune response, altering mosquito breeding environments, and the introduction of new bacteria to mosquitoes.
Natural Anopheles Gambiae Immune Response
One possible avenue for controlling the spread of Plasmodium falciparum is by way of the natural A. gambiae immune response. When Plasmodium falciparum enters a mosquito, there is a systematic response, specific to the type of Plasmodium species infecting the mosquito5. A study in 2002 revealed that the presence of P. falciparum actually regulates and triggers the amount of defensive gene expression occuring in mosquitoes5. The different immune responses that occur are specific to the stage of the Plasmodium falciparum infection. One of the components of the immune response system is transmembrane PGN Recognition Protein LC, which is a peptidoglycan receptor that senses the presence of infections in a mosquito's blood or gut. It is able to activate a signaling pathway that drastically reduces the proliferation of microbes in mosquito guts, along with infections from P. falciparum6. An understanding of the details of mosquito immune responses to Plasmodium falciparum could be used to produce mosquitoes that are resistant to infection, in order to fight the spread of Plasmodium falciparum.
Environmental Influences on Mosquito Vectors
The environment in which mosquitoes breed is influential in tandem with the natural mosquito immune response, against Plasmodium falciparum infections7. The variety of bacterial flora in different A. gambiae mosquito guts is a direct result of their breeding sites, and the composition of the gut microbiota inside mosquitoes has been shown to directly impact their vectorial capacity78. Mosquitoes that have had their gut bacterial flora removed with antibiotics are more succeptible to infection by P. falciparum than those whose gut flora remain in tact8. The presence of microbes in a mosquito's gut help to create immune cells including anti-Plasmodium factors which may inhibit the development of Plasmodium falciparum8..
Introduction of Bacteria A. gambiae
The Wolbachia pipientis bacteria, shown on the right, normally infect many arthropod species. Wolbachia pipientis bacteria do not infect Anopheles gambiae in nature. The bacteria were introduced experimentally to the Anopheles gambiae mosquitoes to test whether they could be used to control the spread P. falciparum. Experiments showed that virulent strains of the bacteria can survive and replicate when injected into a mosquito, demonstrating that viable infections are possible9. The result is hopeful, since the artificial infection of mosquitoes with the Wolbachia pipientis bacteria can negatively affect the development of malaria, as well as the mosquito population itself9. However, the use of Wolbachia pipientis to control P. falciparum is dependent on successful vertical transmission of Wolbachia pipientis in A. gambiae, which has yet to be tested.
Conclusion
Malaria is a persistent and dangerous infectious disease. By studying the most dangerous and influential strain of malaria-causing parasite, Plasmodium falciparum, researchers have been able to better understand the mechanism of spread through Anopheles gambiae, and discover new possible approaches for malaria control. As details are revealed about the specific mechanisms of the mosquito immune system, more control strategies present themselves. The viability of the use of the innate mosquito immune responses, the modification of environments in which mosquitoes thrive, and the artificial introduction of bacteria to mosquito populations in the fight against Plasmodium falciparum are unclear as of yet56789. Further research and testing is needed to discover effective control methods that can be implemented on large populations and prevent devastating malaria outbreaks in the future.
References
1. Kaiser Family Foundation. "The Global Malaria Epidemic" Fact Sheet. November 2012.
2. Perlmann, P., and M. Troye-Blomberg. "Malaria blood-stage infection and its control by the immune system." Folia biologica 46.6 (2000): 210.
3. Gardner, Malcolm J., et al. "Genome sequence of the human malaria parasite Plasmodium falciparum." Nature 419.6906 (2002): 498-511. <br
4. Koella, Jacob C., Flemming L. SÖrensen, and R. A. Anderson. "The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae." Proceedings of the Royal Society of London. Series B: Biological Sciences 265.1398 (1998): 763-768.
5. Tahar, Rachida, et al. "Immune response of Anopheles gambiae to the early sporogonic stages of the human malaria parasite Plasmodium falciparum." The EMBO journal 21.24 (2002): 6673-6680.
6. Meister, Stephan, et al. "Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites." PLoS pathogens 5.8 (2009): e1000542.
7. Boissière, Anne, et al. "Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection." PLoS Pathogens 8.5 (2012): e1002742.
8. Dong, Yue mei, Fabio Manfredini, and George Dimopoulos. "Implication of the mosquito midgut microbiota in the defense against malaria parasites." PLoS pathogens 5.5 (2009): e1000423.
9. Jin, Chaoyang, Xiaoxia Ren, and Jason L. Rasgon. "The virulent Wolbachia strain wMelPop efficiently establishes somatic infections in the malaria vector Anopheles gambiae." Applied and environmental microbiology 75.10 (2009): 3373-3376.
10. "DPDx - Malaria Image Library"
Edited by Lydia de Pillis-Lindheim, a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.
