Plasmodium falciparum: New Developments
Section
By Charley Myers
Introduction
Plasmodium falciparum is a protozoan of the eukaryotic domain. It is most widely known in today's world as one of the most common malarial parasites. This particular species causes malignant malaria, which leads to the most complications and mortality rates of any other malaria-causing agent. [1]
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Sample citations: [2]
[3]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Background
Plasmodium falciparum is a protozoan of the eukaryotic domain. It is widely known in today's world as one of the most common malarial parasites. This particular species causes malignant malaria, which leads to the most complications and mortality rates of all malaria-causing agents. It is estimated that between 300 million and 500 million people are afflicted with malaria annually (WHO). The majority of these incidences of malaria occur in sub-Saharan Africa and affect children under 5. According to the CDC, there are 156 species of Plasmodium, four of which are considered parasitic to humans. These include P. falciparum, P. vivax, P. ovale and P. malaria. Plasmodium falciparum has the highest rate of malarial infection among the four species [4]. We know for a fact that P. falciparum is a malarial parasite that targets humans. What is relatively unknown, however, is the exact mechanism by which the protozoa are able to enter the cell and cause disease.
The Physical Microbe
Taxonomy
Kingdom: Protista Subkingdom: Protozoa Phylum: Apicomplexa Class: Sporozoasida Order: Eucoccidiorida Family: Plasmodiidae Genus: Plasmodium Species: Falciparum
Metabolism
Plasmodium falciparum is a highly proliferating organism whose job is very energetically costly. The protozoa must maintain a high metabolic rate in order to infect its various hosts. It is clear that the metabolism of P. falciparum is closely intertwined with that of its host, as the two share a very intimate relationship. The metabolism of the microbe must adapt to its changing environments. For example, when in the blood stage of its lifecycle, P. falciparum acquires most of its energy from oxidizing glucose into lactate through the process of glycolysis. This parasite utilizes up to 75% times more glucose than its uninfected erythrocyte counterparts[5]. Glycolysis alone is not very productive in the gathering of energy, as it generally only produces 2 ATP per molecule of glucose. A reasonable way to generate more ATP would be to carry out the TCA cycle. However, as the mammalian blood is teeming with glucose, it is efficient enough a process to perform glycolysis alone[6].
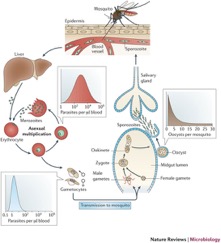
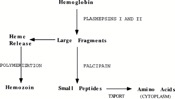
There is evidence that proposes hemoglobin degradation as an additional form of generating energy in P. falciparum, on top of glycolysis. During the intraerythrotic stage of the P. falciparum life cycle, in which the protozoa is parasitizing a vertebrate, the cytoplasm of the host cell is consumed and between 60% and 80% of the hemoglobin in the cell is degraded[7]. After the degradation the heme moiety is stored as a polymer, known as the malaria pigment hemozoin, instead of being recycled[8]. P. falciparum may then utilize this polymer in order to produce amino acids, which are then integrated into parasite proteins and may also be used in metabolism<ref?[7]</ref>. This is a good example of parasitism because the parasite, P. falciparum is pooling the resources of the host (the hemoglobin) and turning them into an integral part of its own survival (amino acids) (Figure 2).
Hemoglobin metabolism is not fully sufficient for the metabolism of P. falciparum. But it can be combined with exogenous amino acid synthesis in order to allow for P. falciparum survival[9]. Knowledge about hemoglobin degradation has proven very useful to researchers targeting malaria outbreaks worldwide. This is because the enzymes that are involved in hemoglobin breakdown, or proteolysis, may be potential targets for antimalarial drug therapy (see section on Malarial Drug Therapy).
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ [1]
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
- ↑ [2]
- ↑ [3]
- ↑ [4]
- ↑ [5]
- ↑ [6]
- ↑ [[[Image:Figure_2_p.fal.jpg|thumb|300px|right|Figure 2. Hemoglobin degradation pathway in P. falciparum. Francis et al 1997.]]]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.