Plasmodium falciparum: New Developments
Section
By Charley Myers
Introduction
Plasmodium falciparum is a protozoan of the eukaryotic domain. It is most widely known in today's world as one of the most common malarial parasites. This particular species causes malignant malaria, which leads to the most complications and mortality rates of any other malaria-causing agent. [1]
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Sample citations: [2]
[3]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Background
Plasmodium falciparum is a protozoan of the eukaryotic domain. It is widely known in today's world as one of the most common malarial parasites. This particular species causes malignant malaria, which leads to the most complications and mortality rates of all malaria-causing agents. It is estimated that between 300 million and 500 million people are afflicted with malaria annually (WHO). The majority of these incidences of malaria occur in sub-Saharan Africa and affect children under 5. According to the CDC, there are 156 species of Plasmodium, four of which are considered parasitic to humans. These include P. falciparum, P. vivax, P. ovale and P. malaria. Plasmodium falciparum has the highest rate of malarial infection among the four species [4]. We know for a fact that P. falciparum is a malarial parasite that targets humans. What is relatively unknown, however, is the exact mechanism by which the protozoa are able to enter the cell and cause disease.
The Physical Microbe
Taxonomy
Kingdom: Protista Subkingdom: Protozoa Phylum: Apicomplexa Class: Sporozoasida Order: Eucoccidiorida Family: Plasmodiidae Genus: Plasmodium Species: Falciparum
Metabolism
Plasmodium falciparum is a highly proliferating organism whose job is very energetically costly. The protozoa must maintain a high metabolic rate in order to infect its various hosts. It is clear that the metabolism of P. falciparum is closely intertwined with that of its host, as the two share a very intimate relationship. The metabolism of the microbe must adapt to its changing environments. For example, when in the blood stage of its lifecycle, P. falciparum acquires most of its energy from oxidizing glucose into lactate through the process of glycolysis. This parasite utilizes up to 75% times more glucose than its uninfected erythrocyte counterparts[5]. Glycolysis alone is not very productive in the gathering of energy, as it generally only produces 2 ATP per molecule of glucose. A reasonable way to generate more ATP would be to carry out the TCA cycle. However, as the mammalian blood is teeming with glucose, it is efficient enough a process to perform glycolysis alone[6].
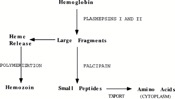
There is evidence that proposes hemoglobin degradation as an additional form of generating energy in P. falciparum, on top of glycolysis. During the intraerythrotic stage of the P. falciparum life cycle, in which the protozoa is parasitizing a vertebrate, the cytoplasm of the host cell is consumed and between 60% and 80% of the hemoglobin in the cell is degraded[7]. After the degradation the heme moiety is stored as a polymer, known as the malaria pigment hemozoin, instead of being recycled[8]. P. falciparum may then utilize this polymer in order to produce amino acids, which are then integrated into parasite proteins and may also be used in metabolism[9]. This is a good example of parasitism because the parasite, P. falciparum is pooling the resources of the host (the hemoglobin) and turning them into an integral part of its own survival (amino acids) (Figure 2).
Hemoglobin metabolism is not fully sufficient for the metabolism of P. falciparum. But it can be combined with exogenous amino acid synthesis in order to allow for P. falciparum survival[10]. Knowledge about hemoglobin degradation has proven very useful to researchers targeting malaria outbreaks worldwide. This is because the enzymes that are involved in hemoglobin breakdown, or proteolysis, may be potential targets for antimalarial drug therapy (see section on Malarial Drug Therapy).
Life Cycle and Transmission
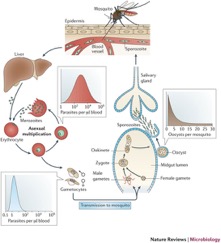
Plasmodium falciparum have a complicated life cycle. They are able to produce both sexually and asexually, although may only do so at different times during the cycle. To carry out the asexual cycle, the parasite must have a vertebrate host, whereas a female Anopheles mosquito is needed for the completion of the sexual cycle[11]. Transmission of P. falciparum, and therefore malaria, begins when the parasite is in the sexual stage of its life cycle. A female Anopheles mosquito injects P. falciparum sporozoites into the vertebrate bloodstream during a blood meal[12]. Human infection begins in the liver, as these sporozoites travel to the liver and invade the hepatocytes. This stage is known as pre-erythrocytic development[13]. Once this is complete, the asexual stage of P. falciparum’s life cycle can begin via the release of merozoites into the host bloodstream (Figure 3).
Some of these merozoites mature into sexual gametocytes, which is the only vector through which transmission from humans to mosquitos may occur[14]. The remaining gametocytes, those of stages I-IV are sequestered into the bone marrow while stage V gametocytes may circulate freely in the peripheral blood. Once these mature (Stage V) gametocytes are ingested by a female mosquito, gamete fusion occurs in the midgut of the mosquito and produces a mobile ookinete which can form oocysts in the midgut wall[15]. As time goes on, these oocysts grow and mature and eventually burst, releasing sporozoites that travel to the salivary glands of the mosquito. These sporozoites then go on to infect humans during a blood meal, starting the cycle again.
Transmission of malaria can vary by environment. For example, areas in which the vector (Anopheles) has ample water habitats and therefore longer life spans, giving the parasite more time to mature, see higher intensity of transmission[16]. Climactic conditions, such as rainfall, temperature and humidity, also affect transmission of P. falciparum to humans. Transmission is often of a seasonal nature, with peaks in intensity during and immediately after the rainy season[17]. Anopheles, especially those found in Africa, prefer to lay their eggs in shallow freshwater, such as puddles. These reservoirs of standing freshwater are obviously more abundant in the rainy season, leading to the increased rates of transmission seen.
Historical Significance
Malaria is not a 21st century problem; Plasmodium falciparum has been problematic for thousands of years. Additionally, it was not always concentrated in Africa, as it might be easy to assume today. In fact, the bustling metropolis of ancient Rome had some of the highest transmission rates of malaria[18]. Because Rome is not a tropical place and because the infection of P. falciparum was concentrated in young children, there must have been extenuating circumstances which allowed for such prevalence of this disease. Transmission rates and therefore the overall incidence of malaria were exceptionally high in ancient Rome, a condition which is known as hyperendemicity[19]. It is estimated that somewhere between 50 and 75% of inhabitants of ancient Rome carried the malaria parasite P. falciparum[20]. Those who were infected in early life but survived were able to develop a gradual immunity to their local strains of P. falciparum. As a result, instances of infection and illness due to P. falciparum became less and less frequent with age. The above situation seems beneficial for the human hosts of P. falciparum and victims of malaria; a lot of built up resistance should be a good thing. However, imperial Rome saw throngs of adult immigrants moving to the crowded areas of the city, most of whom did not have the necessary resistance to the local strains of P. falciparum. Many accounts from ancient writers emphasize the susceptibility of adult immigrants to malaria. The infections themselves manifested in quite different ways among hosts, with children experiencing different symptoms than adults. This difference even lead to different terminologies for describing the same disease; children experience “quotidian fever” whereas adults suffered from “semitertian fever”[21]. Additionally, certain demographics seemed to be hit harder than others. In particular, small children and adult men, as mentioned above, were frequent sufferers of a “falciparian infection”[22].
How do we know for sure that P. falciparum was the parasite causing these deadly infections in ancient Rome? Researchers were able to answer just this question when, in the 1990s, a large infant cemetery was discovered on the outskirts of Rome. This find was unusual, as babies rarely received grand official burials in ancient Rome. Plasmodium falciparum was extracted from the bones of one of the inhabitants of the cemetery, a 2 to 3-year-old girl (Figure 4).
Scientists were successfully able to amplify a section of ribosomal DNA of P. falciparum via a Polymerase Chain Reaction[23]. This evidence proves that malaria was indeed the cause of death of many in the ancient world.
Conclusion
References
- ↑ [1]
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
- ↑ [2]
- ↑ [3]
- ↑ [4]
- ↑ [5]
- ↑ [6]
- ↑ [7]
- ↑ [8]
- ↑ [9]
- ↑ [10]
- ↑ [11]
- ↑ [[[Image:Figure_3_.jpg|thumb|300px|right|Figure 3. Nature Reviews. Life cycle of P. falciparum in both human host and mosquito vector.]] ]
- ↑ [[[Image:Figure_3_.jpg|thumb|300px|right|Figure 3. Nature Reviews. Life cycle of P. falciparum in both human host and mosquito vector.]] ]
- ↑ [12]
- ↑ [13]
- ↑ [14]
- ↑ [15]
- ↑ [16]
- ↑ [17]
- ↑ [18]
- ↑ [19]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.