Prions and Encephalopathies: Difference between revisions
| Line 27: | Line 27: | ||
==Interspecific Barriers and Predictors of Susceptibility== | ==Interspecific Barriers and Predictors of Susceptibility== | ||
<br>Include some current research in each topic, with at least one figure showing data.<br> | <br>Include some current research in each topic, with at least one figure showing data.<br> | ||
[[Image:FISB.medium.gif|thumb|300px|right|Digital representation of an incorrectly folded prion. Image created by the Mayo Clinic.]] | |||
==Treatment== | ==Treatment== | ||
Revision as of 14:03, 22 April 2013
Introduction
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: r7_prion.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Digital representation of an incorrectly folded prion. Image created by the Mayo Clinic.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
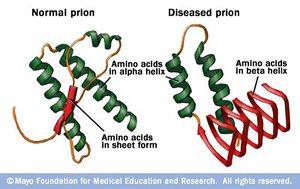
A prion, also known as PrP, is a specific protein, normally found in human and animal tissue. This protein, however, has the ability to change its folding pattern. This alternative folding pattern is thought to cause degenerative diseases in various mammal species. Examples of such diseases are chronic wasting disease in elk and deer, "scrapie" in domesticated sheep, "mad cow disease", transmissible mink encephalopathy, as well as Creutzfeldt-Jakob disease in humans. Autopsies of diseased individuals find concentrated amounts of the prions in nervous system tissues and tissues of the lymphoreticular system - thus it is believed that the abnormally folded protein is the actual infectious agent in these diseases. The normal protein consists of about 253 amino acids and is normally attached to cell membranes by a post-translationally attached glycophosphotidyl-inositol anchor. This protein is sensitive to digestion by proteinase K. In contrast, the abnormal protein is partially resistant to digestion by proteinase K, resulting in a protein which is 60-70 amino acids shorter than the normal.While the true function of the normal PrP is still unsure, recent studies suggest it is linked to programmed cell death mechanisms.1
Transmissible Spongiform Encephalopathies in Animals
Include some current research in each topic, with at least one figure showing data.
The Prion Protein and Mechanism of Infection
Include some current research in each topic, with at least one figure showing data.
Interspecific Barriers and Predictors of Susceptibility
Include some current research in each topic, with at least one figure showing data.
Treatment
Overall paper length should be 3,000 words, with at least 3 figures.
References
[Sample reference] Takai, K., Sugai, A., Itoh, T., and Horikoshi, K. "Palaeococcus ferrophilus gen. nov., sp. nov., a barophilic, hyperthermophilic archaeon from a deep-sea hydrothermal vent chimney". International Journal of Systematic and Evolutionary Microbiology. 2000. Volume 50. p. 489-500. Priola, S., and Vorberg, I. "Molecular Aspects of Disease Pathogenesis in the Transmissible Spongiform Encephalopathies". Methods in Molecular Biology. 2004. Volume 268. p. 517-540.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.