Prions and Encephalopathies: Difference between revisions
| Line 23: | Line 23: | ||
==References== | ==References== | ||
1^Baxter, H., Campbell, G., Whittaker, A., Jones, C., Aitken, A., Simpson, A., Casey, M., Bountiff, L., Gibbard, | 1.^ Baxter, H., Campbell, G., Whittaker, A., Jones, C., Aitken, A., Simpson, A., Casey, M., Bountiff, L., Gibbard, L., and Baxter, R., 2005, Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio-frequency gas-plasma treatment, Journal General Virology, vol 86, pg 2393-2399 | ||
Revision as of 18:33, 23 April 2013
Introduction
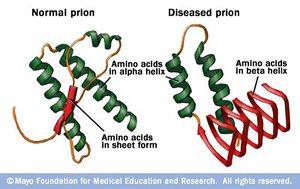
“Prion” is the name given to a class of proteins which can cause severe neurological diseases in a host of mammalian species, including humans. These disorders are a result of misfolding of naturally found proteins in host tissue. While the exact mechanisms of transmission from protein to protein are not completely understood, the general mechanism of infection is that existing misfolded proteins cause the same misfolding in these existing normal proteins. These misfolded proteins are extremely resilient– they are resistant to extreme temperatures, both dry and wet, as well as radiation (Yam, 2011). The accumulation of diseased prions in mammalian tissues, commonly in the nervous system, causes Transmissible Spongiform Encephalopathies (TSEs). This name reflects the appearance of the infected animal’s tissues after infection – the brain and related tissue resemble sponges because of the disintegration of the tissue by the misfolded protein. In general, these disorders are characterized by a long incubation period, followed by an aggressive and always fatal loss of brain and nervous system function. In addition, these diseases do not induce an inflammatory response in the diseased individual (CDC, 2013).2
Transmissible Spongiform Encephalopathies in Animals
Include some current research in each topic, with at least one figure showing data.

The Prion Protein and Mechanism of Infection
Include some current research in each topic, with at least one figure showing data.
Interspecific Barriers and Predictors of Susceptibility
Include some current research in each topic, with at least one figure showing data.
Treatment
Overall paper length should be 3,000 words, with at least 3 figures.
References
1.^ Baxter, H., Campbell, G., Whittaker, A., Jones, C., Aitken, A., Simpson, A., Casey, M., Bountiff, L., Gibbard, L., and Baxter, R., 2005, Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio-frequency gas-plasma treatment, Journal General Virology, vol 86, pg 2393-2399
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.