Propionibacterium acnes: A Teenager’s Worst Nightmare Defined
General Background
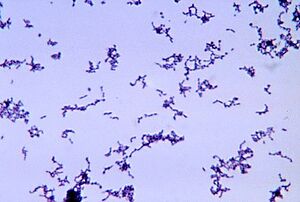
By Megan Lydon
Propionibacterium acnes is a bacterium commonly found on human skin, particularly in sebaceous (oil) glands and hair follicles. It is gram-positive and a fairly slow-growing aerotolerant bacterium. A lower density of P. acnes is detected on the skin of adolescents, especially those prepubescent. The bacteria mainly live on fatty acids. The normal habitat of P. acnes is in the sebaceous follicle shared with the yeast Pityrosporum and aerobic staphylococci and micrococci on its surface [1]. Despite its name, and its colloquial associations, P. acnes is not solely associated with acne [2];it is a normal resident of the skin microbiota in most people. However, it can contribute to the development of acne vulgaris, the most common form of acne, when factors such as excess sebum production, hormonal changes, and inflammation are present. Age-related differences are also noted in which lower levels of P. acnes are found in young children before they hit puberty. Acne is one of the most common skin diseases affecting more than 45 million people in the United States. In addition, in a clinical context, it is estimated that nearly 20% of visits to dermatologists are related to acne and the treatment of the condition [3]. Varying degrees of acne affects nearly all people between the ages of 15-17 with 15-20% of those cases being moderate to severe [4].
As for other opportunistic diseases, P. acnes is known to be involved in endocarditis, osteomyelitis [5][6] arthritis, and postoperative device infection as a result of the insertion of prosthetics and heart valves.
The genome of P. acnes is 2.5 Mb [7]. P. acnes has genes encoding metabolic enzymes allowing it to survive in microaerophilic conditions. When pathogenic, the evasion of host cells (epithelial cells) is characterized by the meditation of the bacterial surface proteins or adhesions recognizing the extracellular matrix (ECM) components. These components are the ideal target of use by many pathogens for tissue colonization. Other skin-associated bacteria such as those in the genera of Staphylococcus and Streptococcus express skin surface proteins known as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that bind to those EMCs. P. acnes is also able to perform this recognition process while adhering to the skin but also is able to promote more reaction by traveling deeper into the skin.
Skin Microbiome
Microbiomes, in general, serve a greater purpose than living organisms just existing in their habitat. Through a combination of commensal species of microbes and their interactions with their habitat, environments are formed where the host and bacteria can adapt and regulate processes either to their advantage or negative effects of competition. The skin microbiome works identically. There is mass variability in the skin microbiome. As for microbes involved, fungi, bacteria, viruses, and small arthropods contribute to this relationship [8].
In addition, the microbiome is much more complex than once thought. Past research has tended to focus only on pathogens and opportunistic pathogens rather than the entire spread of microbes in general (even “harmless” to human hosts). In addition to the variability of the microbes present in the skin microbiome, locations of the skin and their own environments are also variable from person to person. However, even in these differences, homeostasis between the microbiome and host is imperative for continued healthy interactions on the epithelium and to avoid the occurrence of disease. The skin ecosystem is continuously variable in humidity, temperature, pH, and composition of antimicrobial peptides and lipids [9]. In addition, the frequency of hair follicles can also determine the production of sebaceous materials and eccrine/apocrine glands. With this variety of environments, it establishes a separate niche for microbes to fill and thrive in. The abundance of certain bacteria is dependent on these niches [10].
Pathogenesis of P. acnes (Acne Vulgeris)
P. acnes is present on healthy skin and disrupted skin [11]. Therefore associated conditions cannot be classified as infectious diseases. However, pathogenisis of P. acnes does disrupt normal epithelium and its symptoms look to be treated by many. P. acnes is considered to contribute to the development of acne vulgaris, which can chronically affect 15% of people of all ages and at least 85% of teenagers in the United States [12] [13] . As for its role in the disruption of the epithelium is the onset of acne vulgeris. This is a condition where painful, red, and inflamed portions of the skin are infected by P. acnes. P. acnes only triggers the disease when it meets a favorable terrain, therefore the dense colonization of bacteria on the skin is necessary but not sufficient for pathogenesis. Research suggests that the density of bacteria has no effect on the frequency of acne vulgeris however there has been some evidence that certain strains of P. acnes can be more pathogenic when met with favorable conditions. However, it has been shown that there is a correlation between high sebum production and P. acnes density [14]. Regular colonization of P. acnes is quite beneficial to the skin microbiome as it can hydrolyze triglycerides and release free fatty acids to maintain acidic pH on the skin surface. This then helps down regulate the density of other pathogenic bacteria such as Streptococcus pyogenes and Staphylococcus aureus [15].
Follicular Epithelium
The pilosebaceous unit is composed of three subunits: hair follicle, arrector pili muscle, and sebaceous gland [16]. The unit functions mainly as a form of protection against the external environment and aids in the dispersion of sweat. The shape of the hair follicle is also variable and can determine differences in the environments of the skin microbiome. The transformation of the pilosebaceous unit (follicle) into the primary acne lesion is known as “comedogensis”
Public Health Implications
Public Health Implications
Acne Vulgeris Treatments
Acne Vulgeris Treatments
References
- ↑ Leyden, J. J. (1997). Propionibacterium acnes colonization in acne and non-acne.Journal of Investigative Dermatology, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/
- ↑ Bhatia, A., Maisonneuve, J. F., & Persing, D. H. (2004, June). Propionibacterium acnes and chronic diseases. In The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary., Knobler, SL et al.(eds.)(pp. 74-80). From https://www.ncbi.nlm.nih.gov/books/NBK83685/#/
- ↑ Bhatia, A., Maisonneuve, J. F., & Persing, D. H. (2004, June). Propionibacterium acnes and chronic diseases. In The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary., Knobler, SL et al.(eds.)(pp. 74-80). From https://www.ncbi.nlm.nih.gov/books/NBK83685/#/
- ↑ Law, M. P. M., Chuh, A. A. T., Lee, A., & Molinari, N. (2010). Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clinical and experimental dermatology, 35(1), 16-21. From https://academic.oup.com/ced/article-abstract/35/1/16/6622092/
- ↑ Jakab, E., Zbinden, R., Gubler, J., Ruef, C., Von Graevenitz, A., & Krause, M. (1996). Severe infections caused by Propionibacterium acnes: an underestimated pathogen in late postoperative infections. The Yale journal of biology and medicine, 69(6), 477. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2589039//
- ↑ Söderquist, B., Holmberg, A., & Unemo, M. (2010). Propionibacterium acnes as an etiological agent of arthroplastic and osteosynthetic infections–two cases with specific clinical presentation including formation of draining fistulae. Anaerobe, 16(3), 304-306. From https://www.sciencedirect.com/science/article/pii/S107599640900153X/
- ↑ Liu, J., Cheng, A., Bangayan, N. J., Barnard, E., Curd, E., Craft, N., & Li, H. (2014). Draft genome sequences of Propionibacterium acnes type strain ATCC6919 and antibiotic-resistant strain HL411PA1. Genome announcements, 2(4), 10-1128. From https://journals.asm.org/doi/full/10.1128/genomea.00740-14/
- ↑ Leyden, J. J. (1997). Propionibacterium acnes colonization in acne and non-acne.Journal of Investigative Dermatology, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/
- ↑ Leyden, J. J. (1997). Propionibacterium acnes colonization in acne and non-acne.Journal of Investigative Dermatology, 3(108), 379. From https://www.infona.pl/resource/bwmeta1.element.elsevier-c3dc00b6-a2eb-39e6-b2e6-bdac77b2d48c/
- ↑ Grange, P. A., Raingeaud, J., Morelle, W., Marcelin, A. G., Calvez, V., & Dupin, N. (2017). Characterization of a Propionibacterium acnes surface protein as a fibrinogen-binding protein. Scientific Reports, 7(1), 6428. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5527093//
- ↑ Dessinioti, C., & Katsambas, A. D. (2010). The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clinics in dermatology, 28(1), 2-7. https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0738081X09000583?via%3Dihub/
- ↑ Baldwin, H. E., Friedlander, S. F., Eichenfield, L. F., Mancini, A. J., & Yan, A. C. (2011, September). The effects of culture, skin color, and other nonclinical issues on acne treatment. In Seminars in cutaneous medicine and surgery (Vol. 30, No. 3 Suppl, pp. S12-5). From https://europepmc.org/article/med/21943562/
- ↑ Tom, W. L., & Fallon Friedlander, S. (2008). Acne through the ages: case-based observations through childhood and adolescence. Clinical pediatrics, 47(7), 639-651. From https://journals.sagepub.com/doi/abs/10.1177/0009922808315444/
- ↑ Dessinioti, C., & Katsambas, A. D. (2010). The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clinics in dermatology, 28(1), 2-7. https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0738081X09000583?via%3Dihub/
- ↑ Liu, P. F., Hsieh, Y. D., Lin, Y. C., Two, A., Shu, C. W., & Huang, C. M. (2015). Propionibacterium acnes in the pathogenesis and immunotherapy of acne vulgaris. Current Drug Metabolism, 16(4), 245-254. From https://www.ingentaconnect.com/content/ben/cdm/2015/00000016/00000004/art00003/
- ↑ McManus, L. M., & Mitchell, R. N. (2014). Pathobiology of human disease: a dynamic encyclopedia of disease mechanisms. Elsevier. From https://www.sciencedirect.com/referencework/9780123864574/pathobiology-of-human-disease/
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024
