Pseudonocardia sp.: Difference between revisions
| (57 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
==Classification== | ==Classification== | ||
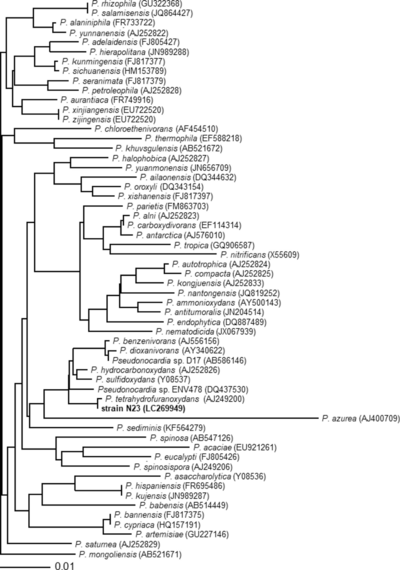
[[File:Phylogeny.png|400px|thumb|right|Phylogenetic tree of ''Pseudonocardia'' species. From Yamamoto, et al. 2018 ''Characterization of newly isolated Pseudonocardia sp. N23 with high 1,4-dioxane-degrading ability'']] | |||
Members of the ''Pseudonocardia'' genus are classified as such: | Members of the ''Pseudonocardia'' genus are classified as such: | ||
| Line 22: | Line 22: | ||
''Pseudonocardia'' are Gram-positive bacteria, meaning they have only one cell membrane and a very thick peptidoglycan cell wall. They are non-motile bacteria, meaning they are unable to move independently in their environments. They are also aerobic bacteria, meaning they use oxygen as a terminal electron acceptor in metabolic pathways. They are rod-shaped and often grow in a branching pattern. | ''Pseudonocardia'' are Gram-positive bacteria, meaning they have only one cell membrane and a very thick peptidoglycan cell wall. They are non-motile bacteria, meaning they are unable to move independently in their environments. They are also aerobic bacteria, meaning they use oxygen as a terminal electron acceptor in metabolic pathways. They are rod-shaped and often grow in a branching pattern. | ||
Many specific ''Pseudonocardia'' species have been isolated from soils, trees, and plant roots from China and Australia, highlighting their importance as environmental bacteria. However, they can also be found in aquatic ecosystems. ''Pseudonardia'' are known as "free-living," meaning they are not dependent on another organism for survival. That being said, ''Pseudonocardia'' species often are part of very significant symbiotic relationships | Many specific ''Pseudonocardia'' species have been isolated from soils, trees, and plant roots from China and Australia, highlighting their importance as environmental bacteria. However, they can also be found in aquatic ecosystems. ''Pseudonardia'' are known as "free-living," meaning they are not dependent on another organism for survival. That being said, ''Pseudonocardia'' species often are part of very significant symbiotic relationships. | ||
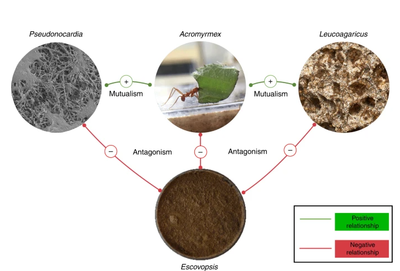
[[File:Symbioses.png|400px|thumb|right|Symbiotic relationships between Attine ants, the fungi they grow, the pathogenic fungi ''Escovopsis'', and ''Pseudonocardia sp.'' From Heine et al., 2018 ''Chemical warfare between leafcutter ant symbionts and a co-evolved pathogen'']] | |||
Attine ants are fungus-growing, leaf-cutting ants. Such ants have their own symbiotic relationships with fungi. These ants grow fungi underground, which they can then eat. In turn, the fungus relies on the ants for its vertical transmission. These fungi are incapable of reproducing sexually using spores, and so ant queens take a piece of the fungal colony with them when they go to establish a new colony, thus propagating the fungus. However, this symbiotic relationship is susceptible to disruption via infection by other fungal species like ''Escovopsis''. This fungus can be extremely pathogenic toward the fungus the ants grow. At the same time that this fungus was discovered, a bacterial symbiont was discovered in the ant that produces compounds that fight infection by organisms like ''Escovopsis''. This bacterium is ''Pseudonocardia''. Compounds naturally produced by ''Pseudonocardia'' are used to protect both the ants and their fungi from infection. These compounds have both antibiotic (bacteria-killing) and antifungal (fungus-killing) properties. Thus, ants having a symbiotic relationship with ''Pseudonocardia'' protects their own symbiotic relationship with the fungi from disruption by infection. | Attine ants are fungus-growing, leaf-cutting ants. Such ants have their own symbiotic relationships with fungi. These ants grow fungi underground, which they can then eat. In turn, the fungus relies on the ants for its vertical transmission. These fungi are incapable of reproducing sexually using spores, and so ant queens take a piece of the fungal colony with them when they go to establish a new colony, thus propagating the fungus. However, this symbiotic relationship is susceptible to disruption via infection by other fungal species like ''Escovopsis''. This fungus can be extremely pathogenic toward the fungus the ants grow. At the same time that this fungus was discovered, a bacterial symbiont was discovered in the ant that produces compounds that fight infection by organisms like ''Escovopsis''. This bacterium is ''Pseudonocardia''. Compounds naturally produced by ''Pseudonocardia'' are used to protect both the ants and their fungi from infection. These compounds have both antibiotic (bacteria-killing) and antifungal (fungus-killing) properties. Thus, ants having a symbiotic relationship with ''Pseudonocardia'' protects their own symbiotic relationship with the fungi from disruption by infection. | ||
| Line 32: | Line 34: | ||
==Cell Structure== | ==Cell Structure== | ||
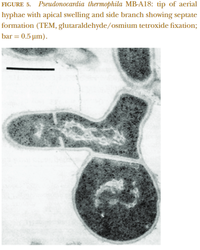
[[File:cellstructureseptation.png|200px|thumb|right|Aerial hyphae showing apical swelling and septate formation. from Bergey's Manual of Systematics of Archaea and Bacteria, Pseudonocardia]] | |||
''Pseudonocardia'' are Gram-positive bacteria, possessing a thick outer layer of peptidoglycan separated from the inner cell membrane by a thin periplasm. Cell membranes are mainly composed of isobranched hexadecanoic acid. ''Pseudonocardia'' are non-acid-fast, meaning they can be decolorized by acid during staining procedures, as they lack mycolic acids in their cell walls. In most species, membrane electron transport is primarily carried out by MK-8(H4), a tetrahydrogenated menaquinone with eight polymerized isoprene units. Determining the molecular composition of cell-envelope components of these species can be helpful in distinguishing them, a process referred to as chemotaxonomy. | ''Pseudonocardia'' are Gram-positive bacteria, possessing a thick outer layer of peptidoglycan separated from the inner cell membrane by a thin periplasm. Cell membranes are mainly composed of isobranched hexadecanoic acid. ''Pseudonocardia'' are non-acid-fast, meaning they can be decolorized by acid during staining procedures, as they lack mycolic acids in their cell walls. In most species, membrane electron transport is primarily carried out by MK-8(H4), a tetrahydrogenated menaquinone with eight polymerized isoprene units. Determining the molecular composition of cell-envelope components of these species can be helpful in distinguishing them, a process referred to as chemotaxonomy. | ||
| Line 39: | Line 43: | ||
==Metabolism== | ==Metabolism== | ||
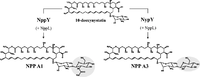
''Pseudonocardia'' is typified by extensive metabolic diversity, with different species able to degrade a wide range of toxic environmental contaminants as well as produce exotic secondary metabolites with antifungal, antibiotic, or other biotechnologically relevant properties. All known species are aerobic, meaning they use diatomic oxygen as a terminal electron acceptor. Most are chemo-organo-heterotrophic, deriving energy from the breakdown of organic compounds, though some are capable of | [[File:nystatinengineered.webp|200px|thumb|left|Polyene antifungals synthesized from the same precursor by wild-type ''Pseudonocardia autotrophica'' (left) and an engineered mutant with an introduced post-PKS enzyme (right). From https://link.springer.com/article/10.1007/s00253-017-8303-8]] | ||
''Pseudonocardia'' is typified by extensive metabolic diversity, with different species able to degrade a wide range of toxic environmental contaminants as well as produce exotic secondary metabolites with antifungal, antibiotic, or other biotechnologically relevant properties. All known species are aerobic, meaning they use diatomic oxygen as a terminal electron acceptor. Most are chemo-organo-heterotrophic, deriving energy from the breakdown of organic compounds, though some are capable of autotrophic growth in certain conditions. A notable number of species in this genus are named for their ability to use atypical compounds as sole sources of carbon for growth; ''Pseudonocardia dioxanivorans'', for example, can fulfill all its carbon and energy needs by breaking down the significant pollutant 1,4-dioxane. Similarly, ''Pseudonocardia carboxydivorans'' can use carbon monoxide (CO) as its only source of carbon, and one species has been identified which grows on the pollutant 1,2,3,5-tetrachlorobenzene as a sole carbon source. Other species are capable of breaking down a wide variety of other chlorinated organic compounds, while others can utilize dimethyl disulfide and dimethyl sulfide, and yet more can break down simple hydrocarbons. Because of this metabolic diversity, as well as their high tolerance to other common pollutants such as tetrahydrofuran (THF), ''Pseudonocardia'' are being actively researched for their potential to remove persistent pollutants from the environment. | |||
In addition to breaking down a diverse set of molecules, ''Pseudonocardia sp'' also produce a wide variety of secondary metabolites, and some species are increasingly used in biotechnology research for their potential to synthesize biologically active compounds. Most notably, ''Pseudonocardia'' species produce many novel antifungal and antibacterial compounds. These form the basis of their mutualism with leafcutter ants, who need these inhibitory chemicals to protect the fungi they cultivate for food against various pathogenic "pests". Multiple classes of novel antifungals have already been isolated from this genus, including the depsipeptide gerumycins and the polyene nystatin/nystatin-like molecules, as well as antibiotics such as branimycins B and C. Many of these compounds display multiple functions, such as various diketopiperazines (DKPs) which exhibit antiviral, antitumor, and antimicrobial activities, while also acting as quorum-sensing molecules for bacteria and signaling molecules for plant growth. | In addition to breaking down a diverse set of molecules, ''Pseudonocardia sp'' also produce a wide variety of secondary metabolites, and some species are increasingly used in biotechnology research for their potential to synthesize biologically active compounds. Most notably, ''Pseudonocardia'' species produce many novel antifungal and antibacterial compounds. These form the basis of their mutualism with leafcutter ants, who need these inhibitory chemicals to protect the fungi they cultivate for food against various pathogenic "pests". Multiple classes of novel antifungals have already been isolated from this genus, including the depsipeptide gerumycins and the polyene nystatin/nystatin-like molecules, as well as antibiotics such as branimycins B and C. Many of these compounds display multiple functions, such as various diketopiperazines (DKPs) which exhibit antiviral, antitumor, and antimicrobial activities, while also acting as quorum-sensing molecules for bacteria and signaling molecules for plant growth. | ||
To fully assess their biotechnological potential, whole-genome characterizations of biosynthetic gene clusters are underway across ''Pseudonocardia''. These studies aim to identify more biologically active secondary metabolites, as well as the enzymes which facilitate their manufacture. The polyketide synthases, for example, are a diverse group of enzymes which synthesize polyene and polyketide compounds, using a complex and highly modular "assembly-line" process. This allows the production of many structurally similar compounds with diverse effects. Novel polyketide synthases which help synthesize nystatin-like compounds have already been isolated from ''Pseudonocardia sp'', and may be useful in bioengineering polyketide biosynthesis pathways to create new compounds. | To fully assess their biotechnological potential, whole-genome characterizations of biosynthetic gene clusters are underway across ''Pseudonocardia''. These studies aim to identify more biologically active secondary metabolites, as well as the enzymes which facilitate their manufacture. The polyketide synthases (PKSs), for example, are a diverse group of enzymes which synthesize polyene and polyketide compounds, using a complex and highly modular "assembly-line" process. This allows the production of many structurally similar compounds with diverse effects. Novel polyketide synthases which help synthesize nystatin-like compounds have already been isolated from ''Pseudonocardia sp'', and may be useful in bioengineering polyketide biosynthesis pathways to create new compounds. | ||
==Habitat and Life Cycle== | |||
''Pseudonocardia sp'' have been isolated from a variety of natural and industrial environments, including plant material, soil, marine sediment, cattle manure, decomposing polyester polyurethane, and biofilter sludge. Many pollutant-catabolizing species have been identified through "enrichment culturing" of industrial sludge samples on media containing a given pollutant as a sole carbon source. ''Pseudonocardia'' are likely to be abundant in hydrogen-rich habitats, as species have been isolated from high-hydrogen environments such as rhizobial nodules or decomposing plant matter, and many species can oxidize dihydrogen and fix carbon dioxide. Furthermore, some species are adapted to break down methoxyphenolic compounds produced through the degradation of lignin--the major structural component of wood--suggesting these organisms are part of a larger system of plant-matter decomposers. However, while ''Pseudonocardia'' have been found in a wide variety of habitats, little is known in detail about their distribution, abundance, or the niches they occupy within these habitats. | |||
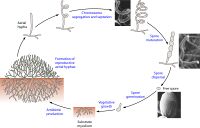
[[File:actinomycetelifecycle.jpg|200px|thumb|left|Life cycle of a typical filamentous, sporulating actinomycete, from spore dispersal to mature spore formation. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4711186/]] | |||
Many ''Pseudonocardia'' species have been researched for their evolutionarily ancient (>50Mya) mutualism with ''Atta'' and ''Acromyrmex'' leafcutter ants. Leafcutter ants cultivate a fungus as a food source, foraging for plant matter from the environment and harvesting it to feed to the fungus. While the basis of this mutualism is not entirely understood, the ''Pseudonocardia'' are known to act as defensive symbionts, manufacturing antimicrobial compounds which protect the cultivated fungus from various fungal and bacterial pathogens. In exchange for protecting the fungal farms, the ants feed their symbionts by secreting nutrients from their exocrine glands, and house them either directly on the ant cuticle or within specialized crypt structures which have convergently evolved multiple times across leafcutter ant lineages. These symbionts are vertically transmitted within ant colonies, though there is also some level of transient acquisition of ''Pseudonocardia'' from the environment, as well as transmission between colonies. Bacterial hyphae grow within the crypt structures as well as out of them, facilitating dispersal across individual ant shells as well as between individuals in a colony. Different strains of ''Pseudonocardia'' severely inhibit one another when growing together on a host, ensuring that only one strain can colonize a given ant colony. | |||
''Pseudonocardia | |||
Regardless of particular habitat, the life of a ''Pseudonocardia'' cell begins as a replicative spore produced by hyphal differentiation of a mature growth of bacterial mycelium. Though these bacteria are non-motile, spores can disperse from aerial hyphae over considerable distance, facilitating spread of ''Pseudonocardia sp'' across large areas. When a spore lands, it begins to divide, entering a "vegetative growth" stage where substrate hyphae grow throughout a nutrient source and begin to pool resources within the hyphae. After a while, aerial hyphae begin to form, growing upward and differentiating into pairs, chains, or single spores. These spores detach upon maturation, starting the process over again. | |||
==Ecology== | ==Ecology== | ||
Pseudonocardia sp. are generally found in a symbiotic relationship with Attine ants of the Atta and Acromyrmex families. These ants are known as leaf-cutting or fungus-growing ants due to their defining characteristic of growing fungi in large farms for consumption. The ants normally grow fungi of the | [[File:pseudonocardia_on_ant.png|200px|thumb|right|(A) ''A. octospinosus'' leafcutter ant bearing symbiotic ''Pseudonocardia'' growths on cuticle tubercles. Bacteria cover the entire body of the ant. Photo courtesy of Alexander L. Wild. (B) Tubercle structures on cuticle of ''A. octospinosus'', showing filamentous growth of symbiotic ''Pseudonocardia'' colonies. (C) Ant tubercles with bacteria removed. From https://www.pnas.org/doi/10.1073/pnas.1809332115]] | ||
While the ants grow and harvest these fungi, their farms are susceptible to infection by another parasitic fungus called Escovopsis. Escovopsis is known to infect the farms in ant colonies and result in massive losses of fungal biomass. This results in a negative effect to the ants who need the farms for nutrition. | |||
In response to the pathogenesis of Escovopsis, the ants have formed a mutualistic relationship with specific strains of Pseudonocardia which grow on a special region on their | ''Pseudonocardia sp.'' are generally found in a symbiotic relationship with Attine ants of the ''Atta'' and ''Acromyrmex'' families. These ants are known as leaf-cutting or fungus-growing ants due to their defining characteristic of growing fungi in large farms for consumption. The ants normally grow fungi of the ''Leucoagaricus'' and ''Leucoprinus'' families, which have evolved to rely of the ants for propagation. | ||
While the ants grow and harvest these fungi, their farms are susceptible to infection by another parasitic fungus called ''Escovopsis''. ''Escovopsis'' is known to infect the farms in ant colonies and result in massive losses of fungal biomass. This results in a negative effect to the ants who need the farms for nutrition. In response to the pathogenesis of ''Escovopsis'', the ants have formed a mutualistic relationship with specific strains of ''Pseudonocardia sp.'' which grow on a special region on their cuticle as a biofilm. The ants provide the ''Pseudonocardia sp.'' with nutrition and protection while the ''Pseudonocardia sp.'' produce anti-fungal compounds that the ants use to protect their farms from ''Escovopsis''. It is still unclear exactly how the mutualism favors the bacterium although it is hypothesized that secretions from the ants glands may be involved as the bacterium are present on special structures on the cuticle of the ant near their endocrine glands. | |||
[[File:Fmicb-11-621041-g001.jpg|200px|thumb|left|''Pseudoncardia'' biofilm on the cuticle of an Attine ant. https://www.frontiersin.org/articles/10.3389/fmicb.2020.621041]] | |||
Studies have shown the antifungal activity of ''Pseudonocardia sp.'' both in-vitro as well as in-vivo on the fungal farms of ants. And while the mechanism of action of the antifungals produced is not yet clearly defined, it has been suggested to have broad antifungal activity. The specificity between the ants and their ''Pseudonocardia'' strains has also been studied. Ants have been shown to be able to recognize their native strain of ''Pseudonocardia'' and have symbiont swaps with non-native strains have resulted in decreased abundance of the bacterium and also an increased infection rate by ''Escovopsis''. ''Pseudonocardia'' has been found on foundress queens during their nuptial flights but not on males, suggesting that the queens may play a role in establishing the symbiosis in new colonies when they establish them. Meanwhile, in established colonies, ''Pseudonocardia'' have been shown to inoculate new ants within two hours of eclosion via contact from other older ants. The symbiotic relationship between ''Pseudonocardia sp.'' and ''Attine'' ants is thought to have established long ago with the relationship having been lost and regained multiple times. The multipartite relationship between Ants, their fungal cultivar, the ''Escocopsis'' parasite and ''Pseudonocardia sp.'' is a classical model to study evolutionary symbiosis. | |||
==References== | ==References== | ||
| Line 77: | Line 93: | ||
11. Structure and mechanism of assembly line polyketide synthases https://sci-hub.se/10.1016/j.sbi.2016.05.009 | 11. Structure and mechanism of assembly line polyketide synthases https://sci-hub.se/10.1016/j.sbi.2016.05.009 | ||
12. Redesign of antifungal polyene glycosylation: engineered biosynthesis of disaccharide-modified NPP https://link.springer.com/article/10.1007/s00253-017-8303-8 | |||
13. Convergent evolution of complex structures for ant–bacterial defensive symbiosis in fungus-farming ants https://www.pnas.org/doi/10.1073/pnas.1809332115 | |||
14. Chemical warfare between leafcutter ant symbionts and a co-evolved pathogen https://www.nature.com/articles/s41467-018-04520-1 | |||
15. Characterization of newly isolated Pseudonocardia sp. N23 with high 1,4-dioxane-degrading ability https://www.researchgate.net/publication/322207537_Characterization_of_newly_isolated_Pseudonocardia_sp_N23_with_high_14-dioxane-degrading_ability | |||
16. Mueller, U. G., Gerardo, N. M., Aanen, D. K., Six, D. L. & Schultz, T. R. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 36, 563–595 (1998). | |||
17. CR Currie, JA Scott, RC Summerbell & D Malloch. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398, 701–704 (1999). | |||
18. Poulsen, M. et al. Variation in Pseudonocardia antibiotic defence helps govern parasite-induced morbidity in Acromyrmex leaf-cutting ants. Environ. Microbiol. Rep. 2, 534–540 (2010). | |||
19. Andersen, S. B., Yek, S. H., Nash, D. R. & Boomsma, J. J. Interaction specificity between leaf-cutting ants and vertically transmitted Pseudonocardia bacteria. BMC Evol. Biol. 15, 1–13 (2015). | |||
20. Li, H. et al. Convergent evolution of complex structures for ant-bacterial defensive symbiosis in fungus-farming ants. Proc. Natl. Acad. Sci. U. S. A. 115, 10720–10725 (2018). | |||
21. Currie, C. R., Marsh, S. E. & Poulsen, M. Interaction between Workers during a Short Time Window Is Required for Bacterial Symbiont Transmission in Acromyrmex Leaf-Cutting Ants. PLoS One 9, (2014). | |||
==Author== | ==Author== | ||
Latest revision as of 18:42, 28 April 2022
Classification
Members of the Pseudonocardia genus are classified as such:
Domain Bacteria
Phylum Actinomycetota
Class Actinomycetia
Order Pseudonocardiales
Family Pseudonocardiaceae
Genus Pseudonocardia
Species
This page covers details about the general genus of Pseudonocardia sp. There are currently 53 members of the Pseudonocardia genus.
Description and Significance
Pseudonocardia are Gram-positive bacteria, meaning they have only one cell membrane and a very thick peptidoglycan cell wall. They are non-motile bacteria, meaning they are unable to move independently in their environments. They are also aerobic bacteria, meaning they use oxygen as a terminal electron acceptor in metabolic pathways. They are rod-shaped and often grow in a branching pattern.
Many specific Pseudonocardia species have been isolated from soils, trees, and plant roots from China and Australia, highlighting their importance as environmental bacteria. However, they can also be found in aquatic ecosystems. Pseudonardia are known as "free-living," meaning they are not dependent on another organism for survival. That being said, Pseudonocardia species often are part of very significant symbiotic relationships.
Attine ants are fungus-growing, leaf-cutting ants. Such ants have their own symbiotic relationships with fungi. These ants grow fungi underground, which they can then eat. In turn, the fungus relies on the ants for its vertical transmission. These fungi are incapable of reproducing sexually using spores, and so ant queens take a piece of the fungal colony with them when they go to establish a new colony, thus propagating the fungus. However, this symbiotic relationship is susceptible to disruption via infection by other fungal species like Escovopsis. This fungus can be extremely pathogenic toward the fungus the ants grow. At the same time that this fungus was discovered, a bacterial symbiont was discovered in the ant that produces compounds that fight infection by organisms like Escovopsis. This bacterium is Pseudonocardia. Compounds naturally produced by Pseudonocardia are used to protect both the ants and their fungi from infection. These compounds have both antibiotic (bacteria-killing) and antifungal (fungus-killing) properties. Thus, ants having a symbiotic relationship with Pseudonocardia protects their own symbiotic relationship with the fungi from disruption by infection.
Genome Structure
Pseudonocardia sp. have one circular chromosome about six million base-pairs (~6,135 kbp, ~6.1 mbp) in length. Pseudonocardia sp. also often have two extrachromosomal plasmids, named pFRP1-1 and pFRP1-2. Both of these plasmids are circular as well. Both are significantly smaller than the genome; pFRP1-1 is about 297 kbp in length while pFRP1-2 is about half of that size, at 121 kbp in length. All in all, the genetic material housed by Pseudonocardia sp. cells code for 5,109 proteins and 63 RNAs. However, because these bacteria live in the soil, they are difficult to isolate and thus there are only about 20 genome sequences currently available for the entire genus, which houses 53 species. DNA G+C content within this genus runs between 68-79%.
pFRP1-1 specifically codes for biosynthetic enzymes to make the antifungal compound gerumycin, which selectively inhibits Escovopsis, the fungal pathogen of the fungal species symbiotic with Attine ants.
Cell Structure
Pseudonocardia are Gram-positive bacteria, possessing a thick outer layer of peptidoglycan separated from the inner cell membrane by a thin periplasm. Cell membranes are mainly composed of isobranched hexadecanoic acid. Pseudonocardia are non-acid-fast, meaning they can be decolorized by acid during staining procedures, as they lack mycolic acids in their cell walls. In most species, membrane electron transport is primarily carried out by MK-8(H4), a tetrahydrogenated menaquinone with eight polymerized isoprene units. Determining the molecular composition of cell-envelope components of these species can be helpful in distinguishing them, a process referred to as chemotaxonomy.
Pseudonocardia are bacilliform, or rod-shaped, bacteria, which grow extensive networks of bacterial mycelium. This mycelium is composed of long, thin filaments called hyphae, which can be divided into "substrate" hyphae that grow throughout a nutrient source and take in nutrients from their surroundings, and "aerial" hyphae that grow upward and facilitate spore dispersal. Within Pseudonocardia, hyphae vary by species in terms of degree of branching, primary mode of growth, and diameter (0.3-2μm). These hyphae often fragment into rod- or oval-shaped elements as they grow, though aerial hyphae of some Pseudonocardia species have been described as squarish.
Growth primarily occurs in this genus through acropetal budding, where new hyphae grow from a bud structure formed by constriction and enlargement of a hyphal tip, or basipetal septation, where existing hyphae are segmented by the growth of interior cell walls called septa. Mature hyphal structures form reproductive spores, which are essentially semi-dormant bacterial cells--aerial hyphae sometimes differentiate into spore chains, though spores usually grow singly or in pairs on substrate and aerial hyphae. In addition to spore growth on acropetal buds or newly septated cells, aging hyphae also irregularly produce spores. Spores vary in size and morphology by species, but are on the order of 0.5-5μm in diameter. Pseudonocardia sp generally grow well on standard media for hyphae-forming actinomycetes, such as Czapek medium or yeast extract agar, usually forming substrate and aerial hyphae which vary in color depending on growth conditions.
Metabolism
Pseudonocardia is typified by extensive metabolic diversity, with different species able to degrade a wide range of toxic environmental contaminants as well as produce exotic secondary metabolites with antifungal, antibiotic, or other biotechnologically relevant properties. All known species are aerobic, meaning they use diatomic oxygen as a terminal electron acceptor. Most are chemo-organo-heterotrophic, deriving energy from the breakdown of organic compounds, though some are capable of autotrophic growth in certain conditions. A notable number of species in this genus are named for their ability to use atypical compounds as sole sources of carbon for growth; Pseudonocardia dioxanivorans, for example, can fulfill all its carbon and energy needs by breaking down the significant pollutant 1,4-dioxane. Similarly, Pseudonocardia carboxydivorans can use carbon monoxide (CO) as its only source of carbon, and one species has been identified which grows on the pollutant 1,2,3,5-tetrachlorobenzene as a sole carbon source. Other species are capable of breaking down a wide variety of other chlorinated organic compounds, while others can utilize dimethyl disulfide and dimethyl sulfide, and yet more can break down simple hydrocarbons. Because of this metabolic diversity, as well as their high tolerance to other common pollutants such as tetrahydrofuran (THF), Pseudonocardia are being actively researched for their potential to remove persistent pollutants from the environment.
In addition to breaking down a diverse set of molecules, Pseudonocardia sp also produce a wide variety of secondary metabolites, and some species are increasingly used in biotechnology research for their potential to synthesize biologically active compounds. Most notably, Pseudonocardia species produce many novel antifungal and antibacterial compounds. These form the basis of their mutualism with leafcutter ants, who need these inhibitory chemicals to protect the fungi they cultivate for food against various pathogenic "pests". Multiple classes of novel antifungals have already been isolated from this genus, including the depsipeptide gerumycins and the polyene nystatin/nystatin-like molecules, as well as antibiotics such as branimycins B and C. Many of these compounds display multiple functions, such as various diketopiperazines (DKPs) which exhibit antiviral, antitumor, and antimicrobial activities, while also acting as quorum-sensing molecules for bacteria and signaling molecules for plant growth.
To fully assess their biotechnological potential, whole-genome characterizations of biosynthetic gene clusters are underway across Pseudonocardia. These studies aim to identify more biologically active secondary metabolites, as well as the enzymes which facilitate their manufacture. The polyketide synthases (PKSs), for example, are a diverse group of enzymes which synthesize polyene and polyketide compounds, using a complex and highly modular "assembly-line" process. This allows the production of many structurally similar compounds with diverse effects. Novel polyketide synthases which help synthesize nystatin-like compounds have already been isolated from Pseudonocardia sp, and may be useful in bioengineering polyketide biosynthesis pathways to create new compounds.
Habitat and Life Cycle
Pseudonocardia sp have been isolated from a variety of natural and industrial environments, including plant material, soil, marine sediment, cattle manure, decomposing polyester polyurethane, and biofilter sludge. Many pollutant-catabolizing species have been identified through "enrichment culturing" of industrial sludge samples on media containing a given pollutant as a sole carbon source. Pseudonocardia are likely to be abundant in hydrogen-rich habitats, as species have been isolated from high-hydrogen environments such as rhizobial nodules or decomposing plant matter, and many species can oxidize dihydrogen and fix carbon dioxide. Furthermore, some species are adapted to break down methoxyphenolic compounds produced through the degradation of lignin--the major structural component of wood--suggesting these organisms are part of a larger system of plant-matter decomposers. However, while Pseudonocardia have been found in a wide variety of habitats, little is known in detail about their distribution, abundance, or the niches they occupy within these habitats.
Many Pseudonocardia species have been researched for their evolutionarily ancient (>50Mya) mutualism with Atta and Acromyrmex leafcutter ants. Leafcutter ants cultivate a fungus as a food source, foraging for plant matter from the environment and harvesting it to feed to the fungus. While the basis of this mutualism is not entirely understood, the Pseudonocardia are known to act as defensive symbionts, manufacturing antimicrobial compounds which protect the cultivated fungus from various fungal and bacterial pathogens. In exchange for protecting the fungal farms, the ants feed their symbionts by secreting nutrients from their exocrine glands, and house them either directly on the ant cuticle or within specialized crypt structures which have convergently evolved multiple times across leafcutter ant lineages. These symbionts are vertically transmitted within ant colonies, though there is also some level of transient acquisition of Pseudonocardia from the environment, as well as transmission between colonies. Bacterial hyphae grow within the crypt structures as well as out of them, facilitating dispersal across individual ant shells as well as between individuals in a colony. Different strains of Pseudonocardia severely inhibit one another when growing together on a host, ensuring that only one strain can colonize a given ant colony.
Regardless of particular habitat, the life of a Pseudonocardia cell begins as a replicative spore produced by hyphal differentiation of a mature growth of bacterial mycelium. Though these bacteria are non-motile, spores can disperse from aerial hyphae over considerable distance, facilitating spread of Pseudonocardia sp across large areas. When a spore lands, it begins to divide, entering a "vegetative growth" stage where substrate hyphae grow throughout a nutrient source and begin to pool resources within the hyphae. After a while, aerial hyphae begin to form, growing upward and differentiating into pairs, chains, or single spores. These spores detach upon maturation, starting the process over again.
Ecology
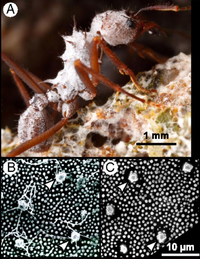
Pseudonocardia sp. are generally found in a symbiotic relationship with Attine ants of the Atta and Acromyrmex families. These ants are known as leaf-cutting or fungus-growing ants due to their defining characteristic of growing fungi in large farms for consumption. The ants normally grow fungi of the Leucoagaricus and Leucoprinus families, which have evolved to rely of the ants for propagation.
While the ants grow and harvest these fungi, their farms are susceptible to infection by another parasitic fungus called Escovopsis. Escovopsis is known to infect the farms in ant colonies and result in massive losses of fungal biomass. This results in a negative effect to the ants who need the farms for nutrition. In response to the pathogenesis of Escovopsis, the ants have formed a mutualistic relationship with specific strains of Pseudonocardia sp. which grow on a special region on their cuticle as a biofilm. The ants provide the Pseudonocardia sp. with nutrition and protection while the Pseudonocardia sp. produce anti-fungal compounds that the ants use to protect their farms from Escovopsis. It is still unclear exactly how the mutualism favors the bacterium although it is hypothesized that secretions from the ants glands may be involved as the bacterium are present on special structures on the cuticle of the ant near their endocrine glands.

Studies have shown the antifungal activity of Pseudonocardia sp. both in-vitro as well as in-vivo on the fungal farms of ants. And while the mechanism of action of the antifungals produced is not yet clearly defined, it has been suggested to have broad antifungal activity. The specificity between the ants and their Pseudonocardia strains has also been studied. Ants have been shown to be able to recognize their native strain of Pseudonocardia and have symbiont swaps with non-native strains have resulted in decreased abundance of the bacterium and also an increased infection rate by Escovopsis. Pseudonocardia has been found on foundress queens during their nuptial flights but not on males, suggesting that the queens may play a role in establishing the symbiosis in new colonies when they establish them. Meanwhile, in established colonies, Pseudonocardia have been shown to inoculate new ants within two hours of eclosion via contact from other older ants. The symbiotic relationship between Pseudonocardia sp. and Attine ants is thought to have established long ago with the relationship having been lost and regained multiple times. The multipartite relationship between Ants, their fungal cultivar, the Escocopsis parasite and Pseudonocardia sp. is a classical model to study evolutionary symbiosis.
References
1. Pseudonocardia https://en.wikipedia.org/wiki/Pseudonocardia
2. Pseudonocardia acaciae https://en.wikipedia.org/wiki/Pseudonocardia_acaciae
3. Pseudonocardia Symbionts of Fungus-Growing Ants and the Evolution of Defensive Secondary Metabolism https://www.frontiersin.org/articles/10.3389/fmicb.2020.621041/full#B50
4. Pseudonocardia sp. EC080625-04 https://www.genome.jp/kegg-bin/show_organism?org=psee
5. Variable genetic architectures produce virtually identical molecules in bacterial symbionts of fungus-growing ants https://www.pnas.org/doi/10.1073/pnas.1515348112
6. Genome Analysis of Two Pseudonocardia Phylotypes Associated with Acromyrmex Leafcutter Ants Reveals Their Biosynthetic Potential https://www.frontiersin.org/articles/10.3389/fmicb.2016.02073/full
7. Taxonomy, Physiology, and Natural Products of Actinobacteria https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4711186/
8. Pseudonocardia, Bergey's Manual of Systematics of Archaea and Bacteria https://sci-hub.se/https://doi.org/10.1002/9781118960608.gbm00184
9. Morphological Identification of Actinobacteria https://www.intechopen.com/chapters/49285
10. Genus Pseudonocardia: What we know about its biological properties, abilities and current application in biotechnology https://sfamjournals.onlinelibrary.wiley.com/doi/pdf/10.1111/jam.15271
11. Structure and mechanism of assembly line polyketide synthases https://sci-hub.se/10.1016/j.sbi.2016.05.009
12. Redesign of antifungal polyene glycosylation: engineered biosynthesis of disaccharide-modified NPP https://link.springer.com/article/10.1007/s00253-017-8303-8
13. Convergent evolution of complex structures for ant–bacterial defensive symbiosis in fungus-farming ants https://www.pnas.org/doi/10.1073/pnas.1809332115
14. Chemical warfare between leafcutter ant symbionts and a co-evolved pathogen https://www.nature.com/articles/s41467-018-04520-1
15. Characterization of newly isolated Pseudonocardia sp. N23 with high 1,4-dioxane-degrading ability https://www.researchgate.net/publication/322207537_Characterization_of_newly_isolated_Pseudonocardia_sp_N23_with_high_14-dioxane-degrading_ability
16. Mueller, U. G., Gerardo, N. M., Aanen, D. K., Six, D. L. & Schultz, T. R. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 36, 563–595 (1998).
17. CR Currie, JA Scott, RC Summerbell & D Malloch. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398, 701–704 (1999).
18. Poulsen, M. et al. Variation in Pseudonocardia antibiotic defence helps govern parasite-induced morbidity in Acromyrmex leaf-cutting ants. Environ. Microbiol. Rep. 2, 534–540 (2010).
19. Andersen, S. B., Yek, S. H., Nash, D. R. & Boomsma, J. J. Interaction specificity between leaf-cutting ants and vertically transmitted Pseudonocardia bacteria. BMC Evol. Biol. 15, 1–13 (2015).
20. Li, H. et al. Convergent evolution of complex structures for ant-bacterial defensive symbiosis in fungus-farming ants. Proc. Natl. Acad. Sci. U. S. A. 115, 10720–10725 (2018).
21. Currie, C. R., Marsh, S. E. & Poulsen, M. Interaction between Workers during a Short Time Window Is Required for Bacterial Symbiont Transmission in Acromyrmex Leaf-Cutting Ants. PLoS One 9, (2014).
Author
Page authored by Caleb Hill, Sannnoong Hu, and Abby Jackson, students of Prof. Jay Lennon at Indiana University.