Reptile-Exotic-Pet-Associated-Salmonellosis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 40: | Line 40: | ||
The main pathway of transmission for <i>Salmonella</i> spp. is via the fecal-oral route usually beginning with the ingestion of contaminated food or water so that the bacterium can reach the intestinal epithelium, spreading throughout the gastrointestinal tract causing gastroenteritis. Humans typically acquire the organisms through direct transmission through handling of a reptile, and via indirect transmission by contact with an object contaminated by a reptile. Other sources of transmission include aquariums, terrariums, cages, as well as the water that most reptiles like turtles and amphibians live in. Clothing that has come into direct contact with reptiles has been associated as a source of transmission, and also bites and claw scratches have been shown to be effective at transmitting <i>Salmonella</i> spp. | The main pathway of transmission for <i>Salmonella</i> spp. is via the fecal-oral route usually beginning with the ingestion of contaminated food or water so that the bacterium can reach the intestinal epithelium, spreading throughout the gastrointestinal tract causing gastroenteritis. Humans typically acquire the organisms through direct transmission through handling of a reptile, and via indirect transmission by contact with an object contaminated by a reptile. Other sources of transmission include aquariums, terrariums, cages, as well as the water that most reptiles like turtles and amphibians live in. Clothing that has come into direct contact with reptiles has been associated as a source of transmission, and also bites and claw scratches have been shown to be effective at transmitting <i>Salmonella</i> spp. | ||
<br><i>Salmonella</i> has been shown to be able to survive for long periods of time in the environment and especially in those environments where it is wet and warm, and has been isolated for prolonged periods from surfaces contaminated by reptile feces (Centers for Disease Control, Jan 2013). It is of important note to highlight the fact that the CDC found that <i>Salmonella</i> has been reported to “survive 89 days in tap water, 115 days in pond water, within dried reptile feces from cages 6 months after removal of the reptile, and from aquarium water 6 weeks after removal of a turtle (CDC, Jan 2013). These findings alone show the danger associated with such a long living organism and help demonstrate the prevalence of the disease Salmonellosis and the dangers associated with <i>Salmonella</i> | <br><i>Salmonella</i> has been shown to be able to survive for long periods of time in the environment and especially in those environments where it is wet and warm, and has been isolated for prolonged periods from surfaces contaminated by reptile feces (Centers for Disease Control, Jan 2013). It is of important note to highlight the fact that the CDC found that <i>Salmonella</i> has been reported to “survive 89 days in tap water, 115 days in pond water, within dried reptile feces from cages 6 months after removal of the reptile, and from aquarium water 6 weeks after removal of a turtle (CDC, Jan 2013). The disease can be transmitted to other individuals, both human and animal alike in fecal matter, with the organisms being shed continually throughout the duration of the infection. Shedding can last anywhere from several days to several weeks, with the ability of certain individuals to become asymptomatic carriers for several months. It has been shown that non-typhoidal Salmonellosis infection patients can shed the bacteria in their feces for me than a year or more with antibiotic treatment typically prolonging the shedding of these organisms (CDC, Jan 2013). Also, over 90% of reptiles may carry <i>Salmonella</i> and these animals may carry up to five different serotypes, increasing the risk associated with reptiles as a whole.These findings alone show the danger associated with such a long living organism and help demonstrate the prevalence of the disease Salmonellosis and the dangers associated with <i>Salmonella</i> as a world-wide issue. | ||
===Pathology and Immune Response=== | ===Pathology and Immune Response=== | ||
[[Image:TKGF3.medium.gif|thumb|300px|right|Pathogenesis model of Salmonella enterica serovar Typhimurium. From [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3623383/pdf/zcm308.pdf. American Society for Microbiology]]] | [[Image:TKGF3.medium.gif|thumb|300px|right|Pathogenesis model of Salmonella enterica serovar Typhimurium. From [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3623383/pdf/zcm308.pdf. American Society for Microbiology]]] | ||
Revision as of 18:58, 20 April 2015
By Tomas Grant
Introduction
Introduce the topic of your paper. What microorganisms are of interest? Habitat? Applications for medicine and/or environment?
Salmonella spp. are rod-shaped, flagellated, and facultative anaerobic bacteria which belong to the family Enterobacteriaceae, and as apart of the phylum Proteobacteria are Gram-negative. The genus Salmonella consists of two species S. enterica and S. bongori , the majority of the diversity lies within the more than 2,600 servovars of the species S. enterica . The three main servers within this species are (1) S. typhi, the cause of systemic infections and typhoid fever, (2) S. Enteritidis, a major food cause of food poisoning associated with poultry farming, and (3) S. typhimurium, which is not fatal in humans, but may cause gastroenteritis, but also more serious cases including septicemia, meningitis, and subnormal empyema. (Rabsch et, al, “The Zoonotic agent Salmonellosis).
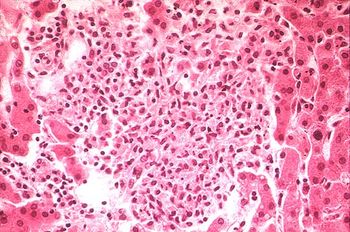
Salmonellosis is one of the most common foodbourne diseases and can be caused by the various serovars of the species S. enterica , and is found worldwide, causing 93.8 million cases of gastroenteritis and over 155,00 deaths annually worldwide (Noellie et el 2014). Understanding the pathology of foodbourne Salmonellosis is important however, there is an increasing concern for Reptile-Exotic-Pet-Associated-Salmonellosis (REPAS ), in which Salmonella serovars act as major zoonotic agents of infection through direct or indirect animal contact in people’s homes, veterinary clinics, farms, zoological gardens, and other professional, public and private settings. (Rabsch et, al, “The Zoonotic agent Salmonellosis) . There are a number of reports that describe the prevalence of Salmonella spp. in reptiles ranging from lizards, snakes, and turtles, and multiple serovars have been identified and shown to be associated with each of these organisms (Sylvester et al, 2014). Reptiles have been shown to be asymptomatic carriers of Salmonella and have been shown to have a natural interaction with the bacteria, with these bacteria being natural inhabitants of reptile gut microflora, shredded at various rates throughout the reptile’s lifecycle. (INSERT) Figure showing prevalence of Salmonella by Species
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Classification
Higher order taxa:
Kingdom:Bacteria
Phylum: Proteobacteria
Order: Gammaproteobacteria
Suborder: Enterobacteriales
Family: Enterobacteriaceae
Genus: Salmonella
Species:
Salmonella enterica subsp. I serovar Typhimurium (S. typhimurium LT2), S. enterica subsp. enterica serovar Typhi (S. typhi CT18), S. enterica subsp. enterica serovar Typhi Ty2 (S. typhi Ty2)
NCBI: Taxonomy Genome: S. typhi CT18 S. typhi Ty2 S. typhimurium|}Epidemiology
Transmission of Salmonella
The main pathway of transmission for Salmonella spp. is via the fecal-oral route usually beginning with the ingestion of contaminated food or water so that the bacterium can reach the intestinal epithelium, spreading throughout the gastrointestinal tract causing gastroenteritis. Humans typically acquire the organisms through direct transmission through handling of a reptile, and via indirect transmission by contact with an object contaminated by a reptile. Other sources of transmission include aquariums, terrariums, cages, as well as the water that most reptiles like turtles and amphibians live in. Clothing that has come into direct contact with reptiles has been associated as a source of transmission, and also bites and claw scratches have been shown to be effective at transmitting Salmonella spp.
Salmonella has been shown to be able to survive for long periods of time in the environment and especially in those environments where it is wet and warm, and has been isolated for prolonged periods from surfaces contaminated by reptile feces (Centers for Disease Control, Jan 2013). It is of important note to highlight the fact that the CDC found that Salmonella has been reported to “survive 89 days in tap water, 115 days in pond water, within dried reptile feces from cages 6 months after removal of the reptile, and from aquarium water 6 weeks after removal of a turtle (CDC, Jan 2013). The disease can be transmitted to other individuals, both human and animal alike in fecal matter, with the organisms being shed continually throughout the duration of the infection. Shedding can last anywhere from several days to several weeks, with the ability of certain individuals to become asymptomatic carriers for several months. It has been shown that non-typhoidal Salmonellosis infection patients can shed the bacteria in their feces for me than a year or more with antibiotic treatment typically prolonging the shedding of these organisms (CDC, Jan 2013). Also, over 90% of reptiles may carry Salmonella and these animals may carry up to five different serotypes, increasing the risk associated with reptiles as a whole.These findings alone show the danger associated with such a long living organism and help demonstrate the prevalence of the disease Salmonellosis and the dangers associated with Salmonella as a world-wide issue.
Pathology and Immune Response
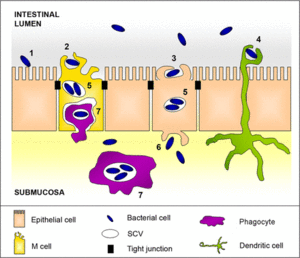
Clinical Signs of Infection
Patients infected by Salmonella typically present a multitude of symptoms including an acute onset of fever, cramping, abdominal pain, diarrhea with or without blood associated with inflammation of the large bowel, as well as nausea and vomiting, but there is a wide spectrum of severity of the illness which may be indicated by only a few or a multitude of symptoms (Fabrega and Vila, 2013). The disease usually manifests after the ingestion of >50,000 bacteria in contaminated food, water, or other sources of infection and non-typhoidal, self-limiting, Salmonella gastroenteritis requires an incubation period ranging from 6 to 72 hours depending on susceptibility and inoculum (CDC Jan 2013). Whether or not Salmonella remains within the intestine or disseminates to other major organs depends on host immune response as well as the virulence of the strain.
Pathogenesis of Salmonella serovar S. Typhimurium
The pathogenesis triggered by the Salmonella serovar S. Typhimurium has been studied extensively and knowledge about its multitude of virulence mechanisms for successfully invading host tissue cells has been compiled. These organisms have a large armamentarium of virulence factors all regulated by an extremely complicated regulatory network, responsible for coordinating and synchronizing all the elements involved. This regulatory mechanism is important because it guarantees the expression of each individual virulence element, but it also facilitates a cross talk between all of the determinants, ensuring the bacteria responds appropriately at all stages in a temporal hierarchal method. These elements include Salmonella pathogenicity islands (SPI’s), as well as other virulence determinants, such as those encoding with the pSLT virulence plasmid, adhesions, flagella, and biofilm-related proteins which all contribute to several stages of the disease (Fabrega and Vila, 2013).
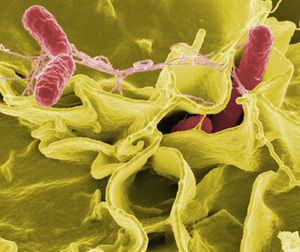
The first step in the infection pathway of Salmonella Typhimurium involves ingestion of the organisms in contaminated food or water as mentioned before, and the subsequent invasion of the stomach which presents the first obstacle to successful infection: the highly acidic pH of the host organism’s stomach. In order to overcome this obstacle, S. Typhimurium activates the acid tolerance response (ATR), which is responsible for providing an inducible pH-homeostatic function in order to maintain the intracellular pH at values higher than those of the extracellular environment (Fabrega and Vila, 2013). Upon entry into the small bowel the organism must reach and then traverse the intestinal mucus layer, the second line of defense against pathogens, and then must adhere to intestinal epithelial cells, causing the appearance of the infection in the form of large cytoskeletal rearrangements within host tissue cells. An important feature of these bacteria are the presence of flagella which gives Salmonella an increased chance at encountering the intestinal epithelium, leading to subsequent adhesion and invasion of host cell tissues. The intestinal epithelium is the primary interface between the internal and external environment, and the adhesion of Salmonella is a key factor in its pathogenicity, or its ability to cause disease. Approach and attachment are enhanced by the motility of the organism and is subsequently driven by several virulence determinants including multiple types of adhesins and fimbriae.
The internal modifications induced within host cells serves to disrupt the normal epithelial brush border and induces the formation of membrane ruffles that engulf the adherent bacteria in large vesicles called Salmonella-containing vacuoles (SCVs) (Fabrega and Vila, 2013). Engulfment of Salmonella is mediated by virulence factors encoded within SP1-I, which encodes for several effector proteins responsible for triggering the invasion of epithelial cells by regulating actin cytoskeletal rearrangements which leads to internalization of the bacteria (Figure1- 2,3,5). (Fabrega and Vila, 2013) These SVC’s are the most important determinants of Salmonella pathogenicity because they represent the only intracellular compartments in which these organisms can survive and replicate,and highlights the facultative intracellular nature of these bacteria. SVC’s are integrated within the early endocytic pathway, but must direct changes within host endocytic trafficking and function to avoid fusion with secondary lysomsomes and delivery of lysosomal enzymes. The bacteria is focused primarily on replication as well as maintenance of the SVC by preventing the delivery of antimicrobial host factors like free-radica; generating complexes. An important mechanism for the maintenance of the integrity of the SVC membrane is the Salmonella induced formation of an F-actin meshwork which surrounds bacterial vacuoles, a process known as vacuole-associated actin polymerization (VAP) (Fabrega and Vila, 2013). This not only protects the bacterial SVC’s, but allows for the migration of these vacuoles to the perinuclear position near the Golgi apparatus, which allows for the interception of host exo- and endo-cytic transport vesicles to obtain nutrients essential for supporting bacterial replication (Fabrega and Vila, 2013).
While initially forming the SVC’s, the invading Salmonella bacteria induce a secretory response in the intestinal epithelium that aims to recruit and transmigrate phagocytes from the submucosal space into the intestinal lumen, followed by the reconstitution of the once disrupted epithelial brush border. Those phagocytes now in the intestinal lumen can be used to engulf individual cells and transport them directly across the membrane. A number of SVC’s also transits to the basolateral membrane and release their internal cells into the submucosa where they are engulfed by phagocytes. More specifically three phagocytic types have been reported to interact directly with invading Salmonella bacteria including (i) neutrophils, (ii) inflammatory monocytes, and (iii) dendritic cells (Fig1-6 and 7) ( (Fabrega and Vila, 2013). These bacteria are then internalized again within an SCV’s allowing for further replication, and the migration of infected phagocytes allows for the dissemination of the invading bacteria via the bloodstream to several additional tissues including the liver, spleen, and in extreme cases the brain.
Antibiotic Resistance and Treatment
Risks Associated With S. enterica infections
Risk Factors
Disease Prevention Measures
Include some current research, with at least one figure showing data.
Include some current research, with at least one figure showing data.
References
[1] Fàbrega, Anna, and Jordi Vila. "Salmonella Enterica Serovar Typhimurium Skills to Succeed in the Host: Virulence and Regulation." Clinical microbiology reviews 26.2 (2013): 308-41.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2015, Kenyon College.
