Rio Tinto (Spain): Difference between revisions
No edit summary |
No edit summary |
||
| Line 41: | Line 41: | ||
==Microbial processes== | ==Microbial processes== | ||
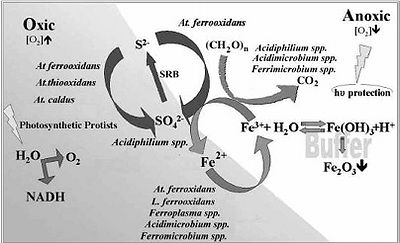
The microbial processes that define this environment are the oxidation of iron and sulfur. Sulfides are the main substrate of choice for the acidic chemolithotrophic organisms present in the Rio Tinto. Sulfides first undergo oxidation threw the polysulfide mechanism which will then produce elemental sulfur. At this state the microbes are able to further oxidize the sulfur into sulfuric acid. This is where the waters high pH stems from. The equations can better help to show what is truly going on. | |||
1)Fe3+ + 3 H2O <-> Fe(OH)3 + 3H+ | |||
2)8MS + 8Fe3+ + 8H+ →8M2+ + 4H2Sn + 8Fe2+ | |||
3)4H2Sn + 8Fe3+ →S08+ 8Fe2+ 8H+ | |||
4)S08+ 12O2 + 8H2O→8SO2− 4 + 16H+ | |||
5)FeS2 + 6Fe3+ + 3H2O→7Fe2+ + S2O2− 3 + 6H+ | |||
6)S2O2− 3 + 8Fe3+ + 5H2O→2SO2− 4 + 8Fe2+ + 10H+ | |||
==Key Microorganisms== | ==Key Microorganisms== | ||
Revision as of 21:56, 11 April 2010
Introduction
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
The Rio Tinto is a river in Southwestern Spain. It is a total of 100km long and flows from the city of Pena de Hierro out to the Atlantic Ocean in Huela. The river is known as an extreme environment due to its low pH and high concentrations of heavy metals. The region has been an important copper mining area for the last 5000 years. It was originally thought that the low pH was caused by the copper mining, but it was later discovered that the low pH numbers are a result of the microbial activity and not of the system itself. The microbial eukaryotic communities show a stronger correlation with the concentration of heavy metals then with the pH of the water. What makes this acidic water system so unique is the fact that it’s primary contributor of biomass comes from eukaryotic micro organisms. It is estimated that up to 65% of total biomass within the system comes from these eukaryotic communities. This is unique because most acidic waters with high metal concentrations are toxic to eukaryotes and limit both growth and biodiversity.
Physical environment
The Rio Tinto is known for its extreme environmental conditions of low pH and high concentrations of heavy metals. On average the pH of the water is around 2, but may very threw out the water system. Some areas measured as low as a 1.1 pH while other areas have recorded pH levels as high as 3. By general observation it has been noticed that the pH tends to be lower in beginning of the river and higher as the river nears the mouth. There are three primary heavy metals found in the water column, they are iron, copper, and zinc. Their averages threw out the year can range from 0.4g/L-20.3g/L for Iron, 0.02g/L-0.7g/L for copper, and 0.02g/L-0.56g/L for zinc. The concentrations of heavy metals within the water column have been found to vary seasonally. In the summer months of June threw September the heavy metal concentrations seem to be the highest. It is thought that this occurs due to that time of year being warmer and being the dry season. This results in lower stream water levels due to less rain and evaporation. These two conditions concentrate the heavy metals within the water system since less water is entering and more is leaving before ever reaching the mouth. The average water temperatures range from 25*C in the summer to 15*C in the winter. As with most aquatic ecosystems the dissolved oxygen levels also very seasonally with the winter months containing higher concentrations of dissolved oxygen when compared to the warmer summer months.
Biological interactions
Microbial processes
The microbial processes that define this environment are the oxidation of iron and sulfur. Sulfides are the main substrate of choice for the acidic chemolithotrophic organisms present in the Rio Tinto. Sulfides first undergo oxidation threw the polysulfide mechanism which will then produce elemental sulfur. At this state the microbes are able to further oxidize the sulfur into sulfuric acid. This is where the waters high pH stems from. The equations can better help to show what is truly going on.
1)Fe3+ + 3 H2O <-> Fe(OH)3 + 3H+ 2)8MS + 8Fe3+ + 8H+ →8M2+ + 4H2Sn + 8Fe2+ 3)4H2Sn + 8Fe3+ →S08+ 8Fe2+ 8H+ 4)S08+ 12O2 + 8H2O→8SO2− 4 + 16H+ 5)FeS2 + 6Fe3+ + 3H2O→7Fe2+ + S2O2− 3 + 6H+ 6)S2O2− 3 + 8Fe3+ + 5H2O→2SO2− 4 + 8Fe2+ + 10H+
Key Microorganisms
Chemolithotrophs
Photosynthetic Primary Producers
Consumers
Decomposers
Examples of organisms within the group
Current Research
Enter summaries of recent research here--at least three required
References
Edited by student of Angela Kent at the University of Illinois at Urbana-Champaign.