Salmonella enterica serovar Paratyphi: Difference between revisions
| Line 26: | Line 26: | ||
===Infectious dose, incubation, and colonization=== | ===Infectious dose, incubation, and colonization=== | ||
The infectious dose of <i>Salmonella</i> Paratyphi is generally greater than 1000 organisms. The incubation period of gastroenteritis caused by <i>Salmonella</i> Paratyphi is between one and ten days, however the incubation period for enteric fever caused by <i>Salmonella</i> | The infectious dose of <i>Salmonella</i> Paratyphi is generally greater than 1000 organisms. The incubation period of gastroenteritis caused by <i>Salmonella</i> Paratyphi is between one and ten days, however the incubation period for enteric fever caused by <i>Salmonella</i> Paratyphi is longer, lasting between one and three weeks. Colonization of the intestines by <i>Salmonella</i> Paratyphi can be buffered by stomach acidity, in which case greater numbers of the bacteria must be ingested in order to result in symptomatic infection. [[#References|[5]]] | ||
===Epidemiology=== | ===Epidemiology=== | ||
Revision as of 16:01, 21 July 2014

Etiology/Bacteriology
Taxonomy
| Domain = Bacteria
| Phylum = Proteobacteria
| Class = Gammaproteobacteria
| Order = Enterobacteriales
| Family = Enterobacteriaceae
| Genus = Salmonella
| species = S. enterica
| subspecies = enterica, serovar Paratyphi A, B, and C
{|NCBI: Taxonomy Genome: Genome|}
Description
Salmonella enterica are motile, non-lactose fermenting, non-sporing, Gram-negative rods, which ferment glucose with production of acid and gas[1] . In the subspecies, enterica, there are three serotypes, Paratyphi A, B, and C. These serotypes are human pathogens that cause paratyphoid fever. Paratyphi A and B are more prominent in causing the disease while Paratyphi C is rare. In the United States, paratyphoid fever is uncommon. Globally, however, an estimated 5.4 million cases occurred in 2000, with outbreaks occurring in East Asia. Salmonella enterica serovar Paratyphi, also referred to as Salmonella Paratyphi, causes 3% of invasive Salmonella infections in the U.S and is correlated to poor sanitation and lack of clean drinking water; therefore, prevention entails basic sanitation and education[2]. Symptoms of paratyphoid fever typically include fever peaking around 40°C, red rash covering the trunk, constipation, severe stomach pain, and loss of appetite. Diagnosing paratyphoid fever is not always the easiest or most simple. Although the disease can sometimes be detected through a simple blood culture, a more accurate confirmation of the disease can be found by a bone marrow culture. Paratyphoid fever and typhoid fever are clinically indistinguishable, thus treatment of the paratyphoid fever is similar to the treatment of typhoid fever. Specific antibiotics can shorten the course of the fever and reduce the chances of death. Paratyphi A infections lead to complications in 10-15% of the cases, such as meningitis, endocarditis, hepatic absess, gall bladder cancer, and pancytopenia[2]. Upon infection by Salmonella Paratyphi, the immune system mounts a humoral response, which is dominated by IgA antibodies. Research indicates that a cell-mediated response is also employed. There are currently no vaccines used to defend against Salmonella Paratyphi ; however, studies are being conducted concerning the efficacy of the Salmonella Typhi Ty21a-vaccine providing protection from Salmonella Paratyphi as well.
Pathogenesis
The pathogenicity of Paratyphoid fever is attributed to several different mechanisms utilized by the Salmonella paratyphi bacterium. Many of the mechanisms of this bacterium are not well understood, but there are several key features of the bacterium's ability to infect and colonize the human host. Salmonella Paratyphi. The bacterium is able to survive the low pH of the stomach and enter the intestines. The mechanism which Salmonella Paratyphi uses in order to adhere to the epithelial cells of the intestines are unknown. Paratyphi B specifically utilizes mechanisms which enable it to cross the intestinal walls and survive in host tissue. A high affinity for iron-uptake may also be important regarding the pathogenicity of this particular bacterium. [3]
Transmission
Salmonella enterica serovars Paratyphi is transmitted primaritly through humans, although rare cases of transmission from domesticated animals has been reported. Most often the pathogen is encountered following ingestion of water contaminated with feces from a person with the disease, or an asymptomatic carrier of the disease. Besides water, milk, raw vegetables, salads, shellfish, and ice can play a role in the transmission of the pathogen if not properly washed or prepared. There have also been rare reports of the disease being transmitted sexually. [4]
Infectious dose, incubation, and colonization
The infectious dose of Salmonella Paratyphi is generally greater than 1000 organisms. The incubation period of gastroenteritis caused by Salmonella Paratyphi is between one and ten days, however the incubation period for enteric fever caused by Salmonella Paratyphi is longer, lasting between one and three weeks. Colonization of the intestines by Salmonella Paratyphi can be buffered by stomach acidity, in which case greater numbers of the bacteria must be ingested in order to result in symptomatic infection. [5]
Epidemiology
Paratyphoid fever generally occurs sporadically or in limited outbreaks. Approximately six million cases of Paratyphoid fever reported annually across the world. In the United States approximately one hundred cases are reported most occurring in recent travelers. The risk of Paratyphi A is most common in South and Southeast Asia. Southern Asia presents the most risk to contracting nalixidic acid resistant paratyphoid fever as well as multidrug resistant paratyphoid fever, which is resistant to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole. [4] [5]
Virulence factors
The virulence factors of Salmonella paratyphi are largely the same as those of other Salmonella enterica serotypes. Two of the major virulence factors include the Salmonella pathogenicity Islands I and II. SPI1 encodes genes which contain a type III secretion system. This system allows the pathogen to inject effector proteins directly into the host cell's cytoplasm, and aids in the bacterial invasion of epithelial cells. SPI2 has roles in helping the bacteria to replicate themselves while within a Salmonella-containing vacuole. SPI2 also encodes for a type III secretion system. [6] [7]
Another important virulence factor, particularly in Paratyphi A. Cytolysin A (ClyA) is a cytotoxic protein which forms pores in membranes. It is a virulence factor most commonly attributed to Escherichia coli, but the gene has been found to be conserved in many Salmonella enterica species. [8]
Several studies have been done to study different plasmids present in Salmonella enterica serotypes. Their role in virulence is not well understood, but studies have shown that Paratyphi C contains a large cryptic plasmid, which is thought to play some role in the virulence of the strain. [6]
Clinical features
Symptoms
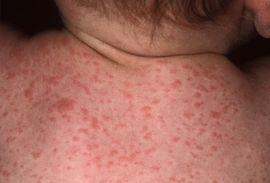
As the name suggests, paratyphoid fever is very similar to typhoid fever and they are often mistaken for one another; however, symptoms of paratyphoid fever have been known to be less severe, as the infection is not nearly as extreme. Symptoms of paratyphoid fever usually occur between 6-30 days after being infected. The most common, and perhaps prominent symptom is severe fatigue accompanied by a fever. The fever typically presents as low-grade and can rise as high as 40°C in the third and fourth days of the illness. Additional symptoms that usually appear are headache, a distinct, red rash all over the trunk, cough, stomach pain, loss of appetite, and constipation or severe diarrhea [9] [10] [11]
Morbidity/Mortality
Once treated, approximately 10% of those with paratyphoid fever will continue to secrete S. paratyphi in their stool for up to three months, which could then be passed on to infect others. Around 2-3% of the recovering patients become permanent carriers for the disease [10]. Paratyphoid fever was once a major cause of mortality throughout the world; however, the infection has begun to be eradicated through various prevention mechanisms. Nevertheless, the disease is still found to be a problem in tropical and underdeveloped countries of the world, typically Africa, Latin America, and South-Eastern Asia. Although the findings are some what inconclusive, it is believed that there is an estimated 5.4 million cases of paratyphoid fever every year [12].
Diagnosis
The diagnosis of paratyphoid fever is usually confirmed by a culture of the blood or bone marrow. As the disease progresses, blood bacterial counts decline, making the blood cultures positive in about only 40-60% of cases. However, the validity of the test can be increased to 80% if multiple sets of blood cultures are taken. The most accurate way to test for paratyphoid fever is by a bone marrow culture because bacterial counts are typically ten times higher in the bone marrow than in a blood culture. Another common way to diagnose the disease is to biopsy the rose-colored rash on the trunk. [10]
Treatment
Paratyphoid fever is treated with specific antibiotics to shorten the course of paratyphoid fever and decreases chances of death. In most parts of the world, a fluoroquinolone is used, which is usually ciprofloxacin. Due to resistance to fluoroquinolones and nalidixic acid in the Indian countries where Paratyphoid fever is more prevalent, cephalosporins are used for treatment. Because multi-drug resistant strains are emerging, azithromycin is more commonly used. After treatment, 3-5 days may be required for the antibiotic to resolve the fever completely. Some patients feel worse during the several days of waiting for the fever to end, however, the height of the fever decreases each day. If the fever does not resolve within 5 days, alternative antibiotics should be considered[13].
Prevention
Risk Avoidance
The incidence of enteric fever is most strongly correlated with poor sanitation and lack of clean drinking water. This is because Salmonella Paratyphi is transmitted through consumption of contaminated food or water. Therefore, in order to decrease the incidence of this disease, it is important to avoid the consumption of contaminated food or water, provide education about food and water safety, and promote basic sanitation. [14] [12]
Immunization
There are currently no vaccines clinically used to defend against Salmonella Paratyphi [12] . Recent studies have indicated that this organism displays evidence of being drug resistant. For example, it was discovered that Salmonella paratyphi has limited sensitivity for ofloxacin, nalidixic acid, and ciprofloxacin. [14]
Because of the evidence for drug resistance, Salmonella Paratyphi poses for a potential health risk; therefore, it is thought that developing an effective vaccine should be of importance. There have been studies on the potential use of an oral vaccine that is used to offer protection against typhoid fever in the protection against Salmonella Paratyphi as well. The oral live Salmonella Typhi Ty21a-vaccine has been shown to offer some protection against Salmonella Paratyphi B, slightly less protection against Salmonella Paratyphi A, and no protection against Salmonella Paratyphi C. [12] These varying levels of protection are due to differences in the shared epitopes of each strain. Despite the research that has been done, there are still no vaccines currently being used in a clinical setting.
Host Immune Response
When the body is infected by Salmonella Paratyphi, a humoral immune response is mounted. The response primarily takes place in the intestinal tract, since the gut is the first line of defense against enteric diseases [15]. Based on research performed with the Ty21a-vaccine, after the host was exposed to the oral vaccine, there was a predominance of IgA antibodies. As part of the immune response, it is believed that after the B cells arrive in the intestinal lining, they undergo isotype switching from IgM to IgA. This specific immunoglobulin isotype is produced to stimulate the destruction of Salmonella bacteria. Other research indicates that a cell-mediated response is also activated upon infection. [12]
References
1 E. Umeh, C. Agbulu. Distribution Pattern Of Salmonella Typhoidal Serotypes In Benue State Central, Nigeria. The Internet Journal of Epidemiology
http://ispub.com/IJE/8/1/5749
2 Sundeep K. Gupta, et al. Laboratory-Based Surveillance of Paratyphoid Fever in the United States: Travel and Antimicrobial Resistance. Clinical Infectious Diseases. 46(11):1656-1663 http://cid.oxfordjournals.org/content/46/11/1656.full
3 Chart, H. The pathogenicity of strains of Salmonella paratyphi B and Salmonella java. Journal of Applied Microbiology. 94:340-348. http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2672.2003.01863.x/pdf
4 Public Health Agency of Canada (PHAC). Salmonella Paratyphi. Infectious Disease Index. http://www.msdsonline.com/resources/msds-resources/free-safety-data-sheet-index/salmonella-paratyphi.aspx
5 Center for Disease Control and Prevention (CDC). Typhoid and Paratyphoid Fever. Traveler’s Health. http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/typhoid-and-paratyphoid-fever
6 Ibarra, J Antonio and Steele-Mortimer, Olivia. Salmonella – the ultimate insider, Salmonella virulence factors that modulate intracellular survival. Cellular Microbiology. 11 (11): 1579-1586. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774479/
7 Lin, Dongxia, Rao, Christopher V., Slauch, James M. The Salmonella SPI1 Type Three Secretion System Responds to Periplasmic Disulfide Bond Status via the Flagellar Apparatus and the RcsCDB System. Journal of Bacteriology. 190(1):87-97. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2223759/
8 Oscarsson, Jan et al. Characterization of a pore-forming cytotoxin expressed by Salmonella enterica Serovars Typhi and Paratyphi A. Infection and Immunity. 70(10): 5759-5769. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC128311/
9 Centers for Disease Control and Prevention (CDC). Infectious Diseases Related to Travel – Typhoid and Paratyphoid Fever. Available at http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/typhoid-and-paratyphoid-fever.
10 White, Nicholas J.: Salmonella typhi and S. paratyphi. Antimicrobe 2010.
11 Government of Western Australia Department of Public Health. Typhoid and Paratyphoid fact sheet. Available at http://www.public.health.wa.gov.au/2/601/2/typhoid_and_paratyphoid_fact_sheet.pm#C, 2014.
12 Pakkanen SH, Kantele JM, Kantele A: Cross-reactive gut-directed immune response against Salmonella enterica serovar Paratyphi A and B in typhoid fever and after oral Ty21a typhoid vaccination. Vaccine 2012, 30: 6047-6053.
13 CDC. Traveler’s Health: Typhoid and Paratyphoid Fever. Available at http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/typhoid-and-paratyphoid-fever
14 Maskey AP, et al: Salmonella enterica Serovar Paratyphi A and S. enterica Serovar Typhi Cause Indistinguishable Clinical Syndromes
in Kathmandu, Nepal. Clinical Infectious Diseases 2006, 42: 1247-53.
15 Rezwanul W, et al: Live Oral Typhoid Vaccine Ty21a Induces Cross-Reactive Humoral Immune Responses against Salmonella enterica Serovar Paratyphi A and S. Paratyphi B in Humans. Clinical and Vaccine Immunology 2012, 19(6): 825-834.
Created by MaKenzi Burke, Madeline Gabe, Regina Swenton, and Jordan Voth, students of Tyrrell Conway at the University of Oklahoma.
