Skin: Difference between revisions
No edit summary |
|||
| (103 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
== | {{Curated}} | ||
The skin is | ==Introduction== | ||
The skin is an organ that contributes to several different epithelial systems as it represents the largest interface between the internal environment of humans and the external world [1,2]. With a mass of approximately five kilograms and it's most superficial layer, known as the stratum corneum, having a surface area generally in the range of 1.75 square meters; the skin is also the largest organ of the human body. The skin serves a number of key functions, with its most basic purposes surrounding its role as a physical barrier protecting the host's internal environment from external pathogens, as well as osmo- and thermoregulation [1,2]. | |||
[[Image:Skinlayers.jpg|thumb|300px|right|Skin layers From the [http://www.nlm.nih.gov/medlineplus/skinconditions.html/ Medline Plus]]] | [[Image:Skinlayers.jpg|thumb|300px|right|Skin layers From the [http://www.nlm.nih.gov/medlineplus/skinconditions.html/ Medline Plus]]] | ||
The microbial fauna colonizing the skin of a healthy human possess a wide and varying lexicon that is distributed across the body in a manner largely dependent on the environmental conditions specific to distinct regions of skin. Important qualities to note that effect the identity of colonizing bacteria include moisture, carbohydrate content, temperature, and pH [8,9]. Generally speaking, three categorical regions of human skin are identified by dermatologists for their microbiological differences: the driest and most basic area being the upper arms and legs; the moistest and most acidic area being the axilla and toewebs; and the area falling in between these two extremes being the face and hands [9]. | |||
=== | ==Description of the Skin's "Environment"== | ||
Skin epithelium functions to keep microbes off the rest | |||
:* Acting as a physical barrier against microbe penetration to tissues underneath | The skin’s key role as the physical barrier delineating between the bloodstream and external environment carries with it many responsibilities. The dermal epithelium is charged with preventing excess water loss, controlling and maintaining its own plasticity and elasticity, and protecting against UV radiation; but also must integrate all these duties in such a way that provides a barrier against the natural microbial proclivity of pathogens to seek out and colonize the skin’s nutrient-rich environment [9]. | ||
:* Secreting a mucus layer so that microbes can not permanently attach to the epithelial cells beneath | |||
:* Shedding or keratinization of the outermost skin cells so microbes are removed from the body | While there is some consistency in the microbial biodiversity across all regions of skin, there are considered to be three general regions of skin that distinguish the specific bacterial flora observed there upon. These regions are (1) the axilla and toewebs; (2) the hands and face; and (3) the upper arms, legs, and trunk. These anatomical distinctions in microbial biodiversity are products of their physical conditions -- specifically, their variation in pH, moisture, body temperature, and concentrations of skin lipids are of particular importance. | ||
Skin epithelium functions to keep microbes off the rest of the body by: | |||
:* Acting as a physical barrier against microbe penetration to tissues underneath | |||
:* Secreting a mucus layer so that microbes can not permanently attach to the epithelial cells beneath | |||
:* Shedding or keratinization of the outermost skin cells so microbes are removed from the body | |||
:* Secreting antimicrobial peptides and proteins to kill off microbes or at least prevent their growth [2]. | :* Secreting antimicrobial peptides and proteins to kill off microbes or at least prevent their growth [2]. | ||
===Physical | ===Physical Qualities of the Skin=== | ||
The composition of the skin’s most superficial layers is such so as to optimize its capacity to prevent bacterial growth on its surface. Examples of this can be found in its relatively high temperature, low carbohydrate and water content, acidic pH, and antimicrobial peptide activities [8]. | |||
The acidic quality of the skin plays an important role in combating bacterial growth. The skin’s pH generally fluctuates between 5.6 and 6.4 depending on the region of the body it is covering, establishing an environment that few species of bacteria are capable of proliferating in [9]. The skin’s acidity results from lactic acid from its own keratinocytic biproducts, amino acids from sweat, and the fatty acids from sebum; as well as the metabolites of the rare microbes capable of colonizing its various surfaces [1]. Although the skin is acidic, it is only suitable for neutrophile growth and not acidophiles or alkaliphiles [1]. | |||
The temperature of the skin varies depending on the location on the body. Toes and fingers tend to have the lowest temperatures, while the axillae and the groin tend to have the highest [1]. The temperatures are usually 25-35 degrees Celsius, which is ideal for mesophiles rather than thermophiles or psychrophiles. The temperature only varies slightly so it there is not a dramatic selection for microbes colonizing certain areas, but does affect the growth rate of the microbes present [1]. | |||
The moisture content on the skin is generally low, which limits the survival and growth of microbes. However, the eccrine glands can produce sweat that increases moisture on the surface of the skin, and can be of particular metabolic value to bacteria in areas where evaporation does not occur easily, such as the toes and axillae [1]. Microbes tend to have greater populations in those occluded areas since there is an accumulation of secretions[1]. | |||
The | The skin generally has a high oxygen concentration, so it acts primarily as an aerobic environment for anaerobes to grow [1,2]. However, the hair follicles inside the skin provide a microaerophillic and/or anaerobic environment that have lower levels of oxygen, so that microaerophiles and obligate anaerobes can also grow [1]. | ||
[[Image:Ml.jpg|thumb|250px|right| An isolated Micrococcus luteus. When M. luteus isn’t present on the skin, it’s found in dust particles. Since it’s located in the air, the amount of air blown can effect its colonization on the skin. From [http://commons.wikimedia.org/wiki/Image:Isolated_bacteria_-_Micrococcus_luteus.jpg Wikimedia Commons Online]]] | |||
===Influence by the Surrounding Environment=== | |||
The skin is always exposed to the external environment. Ecological factors such as climate and habitat have great influence on the microbial flora that colonize the skin. Generally speaking, gram-negative bacilli more frequently colonize regions of the skin with higher moisture content or partial occlusion [9]. Contact with dirt, for example, can introduce non-indigenous or harmful microbes onto the skin. Even air can influence the microbe communities by preventing the airborne microbes from settling on the skin [2]. The openings of the host’s body such as the nose, mouth, urethra, and rectum can also introduce microbes from those regions on to the skin [2]. | |||
The skin is always exposed to the external environment. Contact with dirt, for example, can introduce non-indigenous or harmful microbes onto the skin. Even air can influence the microbe communities by preventing the airborne microbes from settling on the skin [2]. The openings of the host’s body such as the nose, mouth, urethra, and rectum can also introduce microbes from those regions on to the skin [2]. | |||
===Conditions | ===External Conditions that Affect the Skin's Environment=== | ||
Variations in the amount or concentration of sebaceous and sudoriferous glands can affect the skin's temperature, water content, concentration nutrients, osmolarity, pH, and concentration of antimicrobial substances [2]. This is because sebaceous glands are major sources of nutrients for microbes, while the sudoriferous glands produce sweat as a source of water on the skin [2]. Both glands also produce antimicrobial substances important to the skin [2]. | Variations in the amount or concentration of sebaceous and sudoriferous glands can affect the skin's temperature, water content, concentration nutrients, osmolarity, pH, and concentration of antimicrobial substances [2]. This is because sebaceous glands are major sources of nutrients for microbes, while the sudoriferous glands produce sweat as a source of water on the skin [2]. Both glands also produce antimicrobial substances important to the skin [2]. | ||
| Line 38: | Line 46: | ||
Even host characteristics such as age, gender, host’s nutrition, stress, emotional state, disability, hospitalization, personal hygiene, lifestyle, occupation, living conditions, and so on can affect the skin environment[1]. | Even host characteristics such as age, gender, host’s nutrition, stress, emotional state, disability, hospitalization, personal hygiene, lifestyle, occupation, living conditions, and so on can affect the skin environment[1]. | ||
== | ==Bacterial Inhabitants of the Three Categorical Sub-Niches of the Skin== | ||
Microbes are described as “nonpathogenic” if they demonstrate limited growth on the epithelial surface, while those that can invade and have the potential to reach levels of unrestrained growth are defined as “pathogenic” [9]. The majority of the microorganisms that live in the epidermis live in its most superficial layer, known as the stratum corneum, while there are certain species of bacteria that reside in the more secluded areas of follicular canals [8]. | |||
===Axilla and Toewebs: Corynebacterium=== | |||
Corynebacterium is a microbe often found on the skin and is classified as a coryneform. This means that it is non-branching, not-sporing, non-acid-fast, gram-positive, nonmotile and aerobic [1]. | |||
[[Image:240px-Corynebacterium_ulcerans_01.jpg|thumb|350×180px|right|Corynebacterium ulcerans culture on a blood agar plate. From [http://en.wikipedia.org/wiki/Image:Corynebacterium_ulcerans_01.jpg Wikipedia Online Encyclopedia]]] | |||
Corynebacteria can grow at temperatures ranging from 15 to 40 degrees Celsius, but have the best growth at 37 degrees. They are also halotolerant and can grow at NaCl concentrations of up to 10 percent. Because of this, they able to live on locations of the skin with high salt content such as the axillae and in between the toes. These areas are somewhat occluded and therefore have a higher moisture and temperature. These conditions make corynebacteria more prevalent in these regions of the skin. They are found on the skin throughout the body such as the head, arms, legs and hands, but are more prevalent on the lower half [1]. | |||
They are able to use both carbohydrates and amino acids as carbon and energy sources. These macronutrients are readily available in sweat. Glucose is obtained through the hydrolysis of glycoproteins, whereas amino acids are obtained from the hydrolysis of proteins. These amino acids are required for growth. Lipophilic corynebacteria also need other nutrients which they obtain from sweat. These nutrients include riboflavin, thiamine, biotin, nicotinamide, and pantothenate [1]. | |||
====Microbial Interactions of Corynebacterium and its Effects on the Dermal Environment==== | |||
Corynebacteria provide the environment that allows other bacteria to grow. They are able to hydrolyze urea to NH4, which provides a nitrogen source for most cutaneous microbes. At the same time they are dependent on other microbes such as Staphylococcus and Propionibacterium. These types of bacteria provide fatty acids which they obtain from lipids. And in this way they have a complex relationship with these other organisms[1]. | |||
Corynebacterium make up 19% of microbes found on the human skin. And the frequency of detection is much higher in the month of May than in any other time of the year. This is probably due to the increase in average temperature from 8.2 to 16.5 degrees Celsius.(1) | |||
Some species of Corynebacterium, such as Corynebacterium diphtheriae and Corynebacterium ulcerans, are capable of causing skin lesions [12]. These ulcers are often co-infected with Staphylococcus aureus and group A streptococci. These infections can spread to other areas of the body causing pharyngeal infections.(1) Other species like, Corynebacterium tenuis are the cause for trichomycosis axillaris. This is a colonization of the hair shaft in sweat glands in areas such as armpits and pubic area. It consists of a tightly packed overgrowth of this microbe [13]. | |||
===Hands and Face: Propriobacterium acnes=== | |||
Propionibacterium acnes, named for its production of propionic acid, is a commensal gram positive bacterium, found in the sebum produced by sebaceous glands in the skin [14]. It is aerotolerant, allowing it to grow in aerobic conditions but preferring anaerobic fermentation [15]. It tends to grow on hair follicles where there are reduced levels of oxygen and in areas with aerobic organisms that consume oxygen from their immediate environment [16]. Its optimal temperature is 37*C, optimal pH 5.5-6.0. P. acnes is sensitive to UV irradiation but is increasingly antibiotic resistant [17]. It metabolizes fatty acids and sebaceous fluid secreted by the pores they reside in. The products of its fermentation of free short-chained fatty acids assist in its colonization on hair follicles. In addition, they also synthesize proteases, which kill staphylococci and others of the Propionibacteria species. The propionic and acetic acid that it produces are also seen to inhibit growth of other microbial species found in its environment. Although there is no direct benefit to our human skin in harboring such a bacterium, its existence prevents possible onset of more dangerous pathogens, as the worst pathogenic consequences it can bring about are painful lesions and disfiguring acne scars. Its presence occupies the skin such that no dangerously pathogenic microbes can do it harm [19]. | |||
====Microbial Interactions of P. acnes==== | |||
Little beneficial interactions are seen among skin microbes; usually, they are rather antagonistic. However, a beneficial interaction is seen with P. acnes. As one of the more predominant cutaneous microbes, its existence depends on aerobic microbes, including Corynebacterium jeikeium and Acinetobacter, and facilitative microbes, such as staphylococci and Dermabacter hominis, for these microbes consume oxygen and provide P. acnes with their ideal atmosphere to flourish. Without these aerobic and facilitative microbes, they are unable to grow in an aerobic environment. In turn, P. acnes provide other microbes like micrococci and Brevibacteria with propionic and acetic acids, used as carbon sources. Additionally, the bacteria requires vitamins for growth, including biotin, nicotinamide, pantothenate, and thiamine. [[Image:HairFollicle.png|thumb|300 × 277 px|right| A normal hair follicle, location where P. acnes can thrive. From [http://commons.wikimedia.org/wiki/Image:HairFollicle.png Wikimedia Commons Online]]]. Other examples of beneficial interactions include staphylococci producing lactate that is used as a carbon source for Actinobacteria and keratin hydrolysis that produces amino acids used by staphylococci, micrococci, and Brevibacterium [16]. | |||
====Effects of P. acnes on the Dermal Environment==== | |||
Although normally nonpathogenic, P. acnes paves the way for opportunistic pathogens to generate infections. Like most of the skin’s normal flora, it does not pose as a threat until the skin is compromised [19]. An accumulation of P. acnes in sebum-rich areas is implicated in the onset of acnes vulgaris, a skin condition affecting 80% of adolescents in America [20]. P. acnes can bind to oleic acid, which is a major component of sebum. Oleic acid, in turn, promotes co-aggregation, of the organism. This co-aggregation, along with the interaction of the microcolony with oleic acid, would help maintain the organism in the habitat; in this case, hair follicles. Sebum is normally excreted through pores of the skin. Acne, characterized by inflamed lesions, results when sebum and oil builds up under the skin and is further blocked by blackheads [16]. | |||
Though not a direct cause of acnes vulgaris, the presence of P. acnes certainly aids in its onset. Research shows that an acnes inflammation is deemed less severe after treatment with antibiotics that target P. acnes, although P. acnes is becoming increasingly antibiotic resistant [20]. The putative genome of P. acnes has 2333 genes encoding metabolic products including the degradative enzyme lipase that damages host tissue, as well as the heat shock protein that induces an immune response that triggers inflammation [21]. Lipases can also digest excess skin oil and sebum in regions that contain hair follicles and sebaceous glands. Blocked pores create an optimal anaerobic condition for P. acnes to thrive [14]. As P. acnes proliferate in excess, their metabolic products overwhelm the cell wall such that it is ruptured open and a bacterium, such as S. aureus, can cause irritated lesions [16]. | |||
===Upper Arms, Legs, and Trunk: Micrococcus luteus=== | |||
Micrococcus luteus is the most common bacteria of the Micrococcus species found on the skin [22]. The bacteria mostly colonize the legs, arms, and head [24]. These areas of the body do not hold as much moisture as other areas, like the axilla, which explains how Micrococcus luteus tolerate dry conditions. They are mainly innocuous on the skin, although immunodeficiency in the host, like HIV patients, can lead to infection. | |||
[[Image:Micrococcus.jpg|thumb|300px|right|Gram stain of ''Micrococcus'', commonly isolated from the skin. From the [http://textbookofbacteriology.net/normalflora.html/ Todar's Online Textbook of Bacteriology]]] | |||
These gram-positive bacteria are tolerant at high salt concentrations. It’s halotolerant up to 7.5% NaCl, which allows it to tolerate the salinity of the skin- note that seawater is 3% NaCl [16]. Sweat and sebaceous glands greatly contribute to the salinity of the skin. Secretions of sweat from sweat glands to the skin are high in salt concentration and low in water. As the amount of sweating increases, the amount of salt reaching the skin’s surface increases too. The evaporation of water from the release of heat enables the salts to remain present on the skin [25]. As a result of these secretions, nutrients and minerals are present for halotolerant bacteria to survive on the skin. | |||
The nutrients utilized by these bacteria are present on the skin environment. Their primary carbon energy source is derived from amino acids available on the skin after being secreted from sweat glands. These amino acids include arginine, cysteine, methionine, tyrosine, making M. luteus one of the most demanding nutritionally bacteria of the skin [26]. A secondary energy source is the lactic acid secreted from sweat glands to the skin’s surface, which can be converted to its lactate salt form by other bacteria [16]. | |||
====Microbial Interactions of M. luteus==== | |||
M. luteus can find carbon energy sources from the fermentative products of other dermal-colonizing bacteria. Proprionibacterial species – such as P. acnes – produce proprionate and acetate, which is one such potential source of energy; another example is lactate produced by Staphylococcus species [16]. Lactate may be a nutrient for bacteria as well as a contributor to skin pH. The lactate and lactic acid interconvert and contribute to the buffering of the skin pH balance; amphoteric amino acids also contribute to this buffer [25]. As a result, these symbiotic relationships between bacteria are beneficial for providing nutrient sources to each other, allowing many species to inhabit the skin. These bacteria also secrete products to their environment, which keep the skin niche in equilibrium. For example, glycerol is another carbon source used for metabolism in Micrococcus luteus [16]. Lipases are used to degrade oils on the skin into free fatty acids and glycerol. This alcohol generated is metabolized by M. luteus and releases an acid bi-product. Increasing acidity in the environment can contribute to the acidity of the skin pH. This acidity of the skin can be lethal to other microbes that prefer more basic environments. M. luteus therefore contributes to protecting the skin from unwanted microbes by contributing to acidity [16]. | |||
====Effects of M. luteus on the Dermal Environment==== | |||
With the functioning of protease enzymes, many amino acids are released into the environment. Itself along with other neighboring bacteria (like Malassezia species, Staphylococcus species, and Brevibacterium species) benefit from the break down of the proteins so it may acquire free amino acids [16]. Some of the products from the breakdown of these nutrients produce rancid odors [26]. For example, the stench of feet is produced by Micrococcus species breaking down nutrients. These smelly compounds are antibacterial to some bacterial species and can prevent other microbes from inhabiting the skin. | |||
==Non-Bacterial Inhabitants of the Skin== | |||
Genus Fungi, ''Malassezia globosa'' of ''Malassezia'' (Pityrosporum) | |||
[[Image:Bearddandruff.jpg|thumb|231 × 193 px|right| A flake of dandruff, caused by Malassezia globosa. From [http://www.ipedia.net/information/dandruff iPedia.net]]] | |||
One type of fungi that is normally found on the skin is known as Malassezia globosa. M. globosa is a lipophilic, dimorphic yeast that is normally present on the healthy skin of humans [1]. It is most commonly found on host’s skin in tropical environments. It utilizes lipids as a source of carbon and energy, since it is not able to ferment sugars [2]. The lipids contain fatty acids, which M. globosa can use for growth [1]. Because fatty acids and lipids are required for this yeast, it prefers to colonize in areas of the skin that are rich in sebaceous glands such as the scalp, chest, face, and upper back [1]. The yeasts multiply by budding from a scar at the base of the cell, and occur as either spherical or cylindrical forms. Normal skin is mostly saprophytic yeast phase in the spherical form on trunk and oval on the scalp. | |||
Factors like puberty, excessive sweating, warmer season, oil application, malnutrition, and steroids, help in massive growth of the fungus in diseased states. Studies show M. globosa acts as a chemotactic agent for leukocytes inducing inflammation and activation of dermatitis and folliculitis indicating irritant and non-immunogenic stimulant [5]. Although it normally occurs on the skin, it can also demonstrate an invasive etiology that can lead to skin diseases such as pityriasis versicolor, seborrhoeic dermatitis, and dandruff in addition to atopic dermatitis and folliculitis [1 and 2]. The condition known as Pityriasis Capitis, or dandruff, is an inflammatory scalp disorder disrupting cohesion on corneocytes. This is a result of toxin production and lipase activity of the yeast, which stimulates host immune response to the yeast’s antigens [6]. | |||
==Current Research== | |||
1) Molecular Identification of the Malassezia Species [4] | |||
Polymerase Chain Reaction has recently been used to distinguish between the species within the genus ''Malassezia''. Previous techniques for identification of the Malassezia species were based on morphological biochemical, and physiological characteristics that were complex and time consuming. PCR provides a fast and simple method of analyzing the internal transcribed spacer, which varies between species of Malassezia, as a means of differentiation. In this study, four particular Malassezia species which include: M. globosa, M. furfur, M. sympodialasis, and M. restricta that were isolated from dermatitis infections. The specificity of primers were tested and each species of Malassezia had a specific pair primer. The strains of Malassezia were then put into a PCR assay. PCR was able to distinguish between species that were physiologically similar. The PCR method provides an efficient identification system of the malassezia species that can be used in routine practices. | |||
2) Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds [22] | |||
[[Image:Honey.jpg|thumb|180 × 252 px|right| Packaged honey, containing antibiotic properties. From [http://www.ars.usda.gov/is/graphics/photos/k7240-6.htm USDA website. Photo by Scott Bauer]]] | |||
On the human skin bacterial infections can result when they have internal access to invade the host. It usually happens in open wounds or immunodeficient individuals. Treatment of infections has become a challenge since many bacteria evolve to acquire antibiotic resistance. A current study observes the antibiotic properties of honey which may be utilized as an effective antibiotic. Honey has been used since ancient times for healing wounds and respiratory infections, as it is both acidic and possessing high amounts of H2O2, volatiles, and other chemicals that are potentially useful for antibiotics. | |||
The study used different strains of bacteria which potentially cause skin infection. They used honey from an apiary and from honey packaged in barrels. The honey from the apiary was not mixed with other apiaries. However, the packaged honey contained a mix of different sources of honey. The results showed that the honey from the apiaries was more effective in killing bacteria than the packaged honey, and it was believed to be the result of the fact that packaged honey is heated to unpack honeycombs, which may cause the beneficial properties of honey to be lost. One type of bacterium that was effectively targeted was Staphylococcus aureus, which could lead to breakthroughs in the development of antibiotics for future remedies of its increasingly mainstream antibiotic-resistance. | |||
3) Molecular analysis of human forearm superficial skin bacterial biota [10] | |||
Part of the difficulty with defining the bioforal lexicon of the human skin is that there has been little progress made beyond culture-dependent assays in the techniques needed to definitively determine the presence of specific genera of bacteria. These kinds of cultivation methods have been shown to be capable of cultivating less than one percent of all bacterial species. In this experiment by Gao et al., a relatively novel technique called 16S small subunit ribosomal (rRNA) gene surveying was used, which targeted a highly conserved region of the ribosomal genome across most bacterial species with RNA primers to collect a much more representative idea of the true bacterial population on the human skin. | |||
Their analysis focused on samples from the superficial skin from the forearms of six healthy adult subjects, and indeed did demonstrate how truly limited the previous techniques’ data was. Their results showed that three phyla (Actinobacteria, Firmicutes, and Proteobacteria) accounted for 94.6% of all the bacterial clones identified. The results demonstrated that the interpersonal variation in specific bacterial colonization was much greater than previously anticipated, with very low levels of overlap between any of the six subjects. It is the hope of these researchers that these results will help to reveal the identities of populations of microbes that could play important roles in skin diseases. | |||
4) A diversity profile of the human skin microbiota [11] | |||
This experiment by Grice et al. also used the 16S small subunit ribosomal (rRNA) gene surveying technique that Gao used, but instead of analyzing samples from the skin of the forearm, analyzed the inner elbow region of five healthy adults. As this environment is significantly more moist, acidic, and occluded than the forearm used by Gao’s team, it was expected that a different bacterial lexicon would be observed. An additional difference was that besides swabbing the most superficial surface of the skin, this group also analyzed samples from skin scrapings and puncture biopsies, to see if the bacterial populations differed with more invasive skin sample analyses. | |||
The results revealed that the bacterial populations differed little between the superficial swabbing and invasive puncturing samples. This was thought to be a result of the eventual exportation to the surface of all internal bacteria as new skin cells were generated, and not necessarily revealing of any actual consistency in bioflora colonization throughout the epidermis. Similarly to the Gao experiments, Grice’s investigation revealed that a small number of species dominated the skin’s landscapes, with the Proteobacteria pseudomonas species alone representing 59% of the 16S RNA sequences identified in this survey alone. She infers from the conserved nature of this consistency across the individuals that a diseased state could be the product of some alteration in the proportion of these microbes or the introduction of some novel microbe. | |||
5) Vitamin D3 Provides Skin With Protection From Harmful Microbes [28] | |||
In a study led by Dr. Richard Gallo of UCSD School of Medicine, Division of Dermatology, Vitamin D3 is found to be essential as a skin injury heals, protecting a wound from infection and initiating skin repair. When a body lacks or has an insufficient amount of active Vitamin D3, it becomes more vulnerable to microbe infection. The natural immune response when a body is afflicted by an abrasion is to produce an antimicrobial peptide called cathelicidin, which is needed to fight infections. Receptors on white blood cells trigger production of cathelicidin when they sense an invading bacterium. Gallo and his lab have also discovered that skin cells called keratinocytes are stimulated upon injury. They surround a wound and increases Vitamin D3 production. Vitamin D3 aids in expression of genes that identify microbes, such as CD14 and TLR2. These genes form a complex with Vitamin D3, 1,25D3, which triggers more cathelicidin production. In studying mice and human subjects, a decrease in cathelicidin led to an increase in developing infections. 1,25D3 is found to vary between races. For instance, African Americans are found to have a lower concentration of 1,25D3, thus rendering them more susceptible to infection. This may be caused by their body’s inefficient absorption of Vitamin D from sunlight. | |||
This study is ongoing to determine if levels of Vitamin D3 are related to enhanced innate immune defenses. Clinical trials conducted observe both oral and topical Vitamin D in healthy volunteers and patients suffering from antimicrobial peptide production disorders, such as acne and eczema. | |||
6) Newer skin treatments have shown successful prevention of Acne vulgaris [29] | |||
Acne vulgaris is a common skin disease that has many causes. Genetics, hormonal balance, increase in size or excessive activity of the sebaceous gland, cell build up, and bacterial infections. When many of these factors come together, the first stage of acne is formed as microcomedones. When anaerobic conditions are present in microcomedones, P. acnes can overgrow and lead to papules and pustules visible on the skin. Acne has become a problem for many individuals and current skin therapies try to target the factors that cause this disease. | |||
Analysis of current acne treatments have shown successful results. Photodynamic therapy has shown success on anaerobic bacteria. A blue light is used to target bacterial cells, and based on a specific absorption of light it induces P. acnes to produce porphyrin. As a result P.acnes die from this therapy, because this photodynamic reaction creates oxygen presence around the cells. Clinical studies have shown that this technique is effective. Based on such successful trials, this technique will be useful in the future to prevent acne. Cryotherapy uses liquid nitrogen to freeze the skin. The cold is tested to exfoliate and shed parts of the sebaceous gland. As a result less activity of the sebaceous gland is shown, so it reconstructs the skin into a calmer environment. Chemical peels have also been tested to be successful therapies. Chemicals that enhance keratolytic activity are used to restore skin balance. Chemicals like phenol are also used, which have antiseptic properties. | |||
Overall these new therapies have shown successful reduction of acne and scarring. What must be considered in the future is the safety of these procedures. Chemical peels should be regulated to make sure the antiseptic compounds do not harm the skin. Observing the penetration of light to the skin should also be examined in photodynamic therapy for patients. Long term trials will be needed to see if these treatments last and to observe negative developing effects. | |||
==Closing Thoughts== | |||
The skin serves as the human body's most important epithelial interface between the external world of microbial threats and its internal environment. As the body's largest organ, the skin serves to not only physically protect the bloodstream, but also helps with thermoregulation and osmoregulation. Though the skin has evolved to establish itself as a relatively toxic environment for most bacteria to land upon, there are a considerable number of bacteria that have similarly evolved in ways that make them capable of colonizing the nutrient-rich surface that the skin presents. The nutrients and conditions available are not universally consistent throughout the skin; however, as the moisture, pH, temperature, and occlusion varies and creates different niches for different bacteria in different regions of the body. The three specific niches identified and explored here were the moist and acidic axilla and toewebs; the dry and basic upper arms, legs, and trunk; and the intermediate hands and face. The predominant bacteria capable of colonizing these three regions -- as contemporary science is capable of identifying them, at least -- all perform some commensalist or symbiotic function for their host, while benefiting from the ecological niche they are provided with for their own subsistence. | |||
==References== | ==References== | ||
| Line 139: | Line 173: | ||
7. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=18502944 Elizabeth A. Grice, Heidi H. Kong, Gabriel Renaud, Alice C. Young, Gerard G. Bouffard, Robert W. Blakesley, Tyra G. Wolfsberg, Maria L. Turner, and Julia A. Segre. "A diversity profile of the human skin microbiota: NISC Comparative Sequencing Program." Genome Research. Cold Spring Harbor Laboratory Press 2008 July.] | 7. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=18502944 Elizabeth A. Grice, Heidi H. Kong, Gabriel Renaud, Alice C. Young, Gerard G. Bouffard, Robert W. Blakesley, Tyra G. Wolfsberg, Maria L. Turner, and Julia A. Segre. "A diversity profile of the human skin microbiota: NISC Comparative Sequencing Program." Genome Research. Cold Spring Harbor Laboratory Press 2008 July.] | ||
Edited by Patrick A. McGhee, Susan Lin, Eric Pham, Pavithra Ramasubramanian, Deeba Pourmand, | 8. Aly, Raza. Clinical Skin Microbiology. Springfield, IL: Thomas Books, 1987. 11-35. | ||
9. Elias, Peter M., and Kenneth R. Feingold, eds. Skin Barrier. Danbury: Marcel Dekker Incorporated, 2006. | |||
10. [http://www.pnas.org/content/104/8/2927.abstract Gao Z, Tseng CH, Pei Z, Blaser MJ. “Molecular analysis of human forearm superficial skin bacterial biota”. Proceedings of the National Academy of Sciences of the USA. 2007. Volume 104(8). p. 2927-2932.] | |||
11. [http://www.ncbi.nlm.nih.gov/pubmed/18502944 Grice EA, Kong HH, Renaud G, Young AC; NISC Comparative Sequencing Program, Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, Segre JA. “A diversity profile of the human skin microbiota”. Genome Research. 2008. Volume 18(7). p. 1043-1050.] | |||
12. [http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46652008000100011&lng=en&nrm=iso&tlng=en CANTARELLI, Vlademir Vicente, BRODT, Teresa Cristina Z., SECCHI, Carina et al. “Cutaneous infection caused by Corynebacterium pseudodiphtheriticum: a microbiological report.” Rev. Inst. Med. trop. S. Paulo, Jan./Feb. 2008, vol.50, no.1, p.51-52.] | |||
13.[http://www.aafp.org/afp/980515ap/odell.html O'Dell ML (1998). "Skin and wound infections: an overview". Am Fam Physician 57 (10): 2424–32.] | |||
14.Propionibacterium Acnes and Hydrogen Peroxide. Acne Talks. <http://www.acnetalks.com/pimple/Acne-Treatment/Methods/Propionibacterium-Acnes-And-Hydrogen-Peroxide.htm>. Accessed 27 August 2008. | |||
15.Propionibacterium acnes KPA171202 project at Goettingen Genomics Library. NCBI Entrez Genome Project. < http://www.ncbi.nlm.nih.gov/sites/entrez?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=12460>. Accessed 27 August 2008. | |||
16. Wilson, Michael. Microbial Inhabitants of Human: Their Ecology and Role in Health and Disease. Cambridge University Press, 2005. | |||
17. Ingham, Eileen The Immunology of Propionibacterium acnes and Acne. Current Opinion in Infectious Diseases. 1999. Vol. 12(3): p. 191-197. | |||
18. Prater, Alicia Mae. Not All Bacteria Are Bad: Digestion and Immunity Are Aided By Microbes. Suite101, 8 May 2008. < http://bacteriology.suite101.com/article.cfm/not_all_bacteria_are_bad>. Accessed 24 August 2008. | |||
19. Oliver, David. Microbes and You: Normal Flora. The Science Creative Quarterly, Issue Three, Sept07-Apr08. < http://www.scq.ubc.ca/microbes-and-you-normal-flora/>. Accessed on 24 August 2008. | |||
20. Ingram, Eileen, et. al. Inflammation of Acne vulgaris: Failure of Skin Micro-organisms to Modulate Keratinocyte Interleukin 1α Production in vitro. Dermatology 1998; 196:86-88. | |||
21. Brüggemann, Holger et al. The Complete Genome Sequence of Propionibacterium Acnes, a Commensal of Human Skin. Science. 2005. 305: p. 671-672. | |||
22. Basualdo, C., Sgroy, V., Finola, M.S., Marioli, J.M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Veterinary Microbiology. (2007) v. 124, pgs 375-381 | |||
23. Clark, D.J., Hawrylik, S.J., Kavanagh, E., and Opheim D.J. Purification and Characterization of a Unique Alkaline Elastase from Micrococcus luteus. Protein Expression and Purification. (2000) v. 18, pgs 46-55 | |||
24. Kloos*, W.E., and Musselwhite M.S. Distribution and Persistence of Staphylococcus and Micrococcus Species and Other Aerobic Bacteria on Human Species. Applied Microbiology. (1975) v. 30, pg. 381-395 | |||
25. Marples, M. J. The Ecology of the Human Skin. Charles C Thomas Publisher. Springfield, Ill. (1965) pgs103-154 | |||
26. Noble, W.C. The Skin Microflora and Microbial Skin Disease. Cambridge University Press. New York, NY.(1993) pgs 1-70 | |||
27. Slonczewski, J.L., and Foster, J.W. Microbiology, An Evolving Science. W.W. Norton & Company, Inc. New York, NY. (2009) pg 877 | |||
28. Schauber, J. and Gallo, R.L. “Antimicrobial peptides and the skin immune defense system.” Journal of Allergy and Clinical Immunology (2008) v 122, pgs 261-266 | |||
29. Kaszuba A., Bartkowiak R., and Kaszuba A. “Adjunct and relating to a procedure methods of the management of acne vulgaris and traces after treatment” Aesthetic Dermatology (2006) v. 8, pgs 9-13 | |||
Edited by Patrick A. McGhee, Susan Lin, Eric Pham, Pavithra Ramasubramanian, Deeba Pourmand, Wei Chien, Leo Borjon, | |||
David Juma, Cynthia Wong, students of Rachel Larsen | |||
Latest revision as of 15:11, 7 July 2011
Introduction
The skin is an organ that contributes to several different epithelial systems as it represents the largest interface between the internal environment of humans and the external world [1,2]. With a mass of approximately five kilograms and it's most superficial layer, known as the stratum corneum, having a surface area generally in the range of 1.75 square meters; the skin is also the largest organ of the human body. The skin serves a number of key functions, with its most basic purposes surrounding its role as a physical barrier protecting the host's internal environment from external pathogens, as well as osmo- and thermoregulation [1,2].
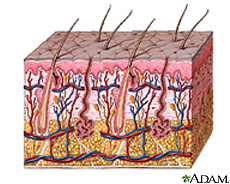
The microbial fauna colonizing the skin of a healthy human possess a wide and varying lexicon that is distributed across the body in a manner largely dependent on the environmental conditions specific to distinct regions of skin. Important qualities to note that effect the identity of colonizing bacteria include moisture, carbohydrate content, temperature, and pH [8,9]. Generally speaking, three categorical regions of human skin are identified by dermatologists for their microbiological differences: the driest and most basic area being the upper arms and legs; the moistest and most acidic area being the axilla and toewebs; and the area falling in between these two extremes being the face and hands [9].
Description of the Skin's "Environment"
The skin’s key role as the physical barrier delineating between the bloodstream and external environment carries with it many responsibilities. The dermal epithelium is charged with preventing excess water loss, controlling and maintaining its own plasticity and elasticity, and protecting against UV radiation; but also must integrate all these duties in such a way that provides a barrier against the natural microbial proclivity of pathogens to seek out and colonize the skin’s nutrient-rich environment [9].
While there is some consistency in the microbial biodiversity across all regions of skin, there are considered to be three general regions of skin that distinguish the specific bacterial flora observed there upon. These regions are (1) the axilla and toewebs; (2) the hands and face; and (3) the upper arms, legs, and trunk. These anatomical distinctions in microbial biodiversity are products of their physical conditions -- specifically, their variation in pH, moisture, body temperature, and concentrations of skin lipids are of particular importance.
Skin epithelium functions to keep microbes off the rest of the body by:
- Acting as a physical barrier against microbe penetration to tissues underneath
- Secreting a mucus layer so that microbes can not permanently attach to the epithelial cells beneath
- Shedding or keratinization of the outermost skin cells so microbes are removed from the body
- Secreting antimicrobial peptides and proteins to kill off microbes or at least prevent their growth [2].
Physical Qualities of the Skin
The composition of the skin’s most superficial layers is such so as to optimize its capacity to prevent bacterial growth on its surface. Examples of this can be found in its relatively high temperature, low carbohydrate and water content, acidic pH, and antimicrobial peptide activities [8].
The acidic quality of the skin plays an important role in combating bacterial growth. The skin’s pH generally fluctuates between 5.6 and 6.4 depending on the region of the body it is covering, establishing an environment that few species of bacteria are capable of proliferating in [9]. The skin’s acidity results from lactic acid from its own keratinocytic biproducts, amino acids from sweat, and the fatty acids from sebum; as well as the metabolites of the rare microbes capable of colonizing its various surfaces [1]. Although the skin is acidic, it is only suitable for neutrophile growth and not acidophiles or alkaliphiles [1].
The temperature of the skin varies depending on the location on the body. Toes and fingers tend to have the lowest temperatures, while the axillae and the groin tend to have the highest [1]. The temperatures are usually 25-35 degrees Celsius, which is ideal for mesophiles rather than thermophiles or psychrophiles. The temperature only varies slightly so it there is not a dramatic selection for microbes colonizing certain areas, but does affect the growth rate of the microbes present [1].
The moisture content on the skin is generally low, which limits the survival and growth of microbes. However, the eccrine glands can produce sweat that increases moisture on the surface of the skin, and can be of particular metabolic value to bacteria in areas where evaporation does not occur easily, such as the toes and axillae [1]. Microbes tend to have greater populations in those occluded areas since there is an accumulation of secretions[1].
The skin generally has a high oxygen concentration, so it acts primarily as an aerobic environment for anaerobes to grow [1,2]. However, the hair follicles inside the skin provide a microaerophillic and/or anaerobic environment that have lower levels of oxygen, so that microaerophiles and obligate anaerobes can also grow [1].
Influence by the Surrounding Environment
The skin is always exposed to the external environment. Ecological factors such as climate and habitat have great influence on the microbial flora that colonize the skin. Generally speaking, gram-negative bacilli more frequently colonize regions of the skin with higher moisture content or partial occlusion [9]. Contact with dirt, for example, can introduce non-indigenous or harmful microbes onto the skin. Even air can influence the microbe communities by preventing the airborne microbes from settling on the skin [2]. The openings of the host’s body such as the nose, mouth, urethra, and rectum can also introduce microbes from those regions on to the skin [2].
External Conditions that Affect the Skin's Environment
Variations in the amount or concentration of sebaceous and sudoriferous glands can affect the skin's temperature, water content, concentration nutrients, osmolarity, pH, and concentration of antimicrobial substances [2]. This is because sebaceous glands are major sources of nutrients for microbes, while the sudoriferous glands produce sweat as a source of water on the skin [2]. Both glands also produce antimicrobial substances important to the skin [2].
Clothing, air-conditioning, or housing, for example, can be considered forms of protection against extreme environments. However, the conjunctiva and exposed regions of the skin have greater fluctuations in temp, humidity, and so forth, in comparison to other bodily systems [1]. Covering areas of the skin, for instance, can prevent the evaporation of water and encourage a build up of secretions and alter the pH [2].
Even host characteristics such as age, gender, host’s nutrition, stress, emotional state, disability, hospitalization, personal hygiene, lifestyle, occupation, living conditions, and so on can affect the skin environment[1].
Bacterial Inhabitants of the Three Categorical Sub-Niches of the Skin
Microbes are described as “nonpathogenic” if they demonstrate limited growth on the epithelial surface, while those that can invade and have the potential to reach levels of unrestrained growth are defined as “pathogenic” [9]. The majority of the microorganisms that live in the epidermis live in its most superficial layer, known as the stratum corneum, while there are certain species of bacteria that reside in the more secluded areas of follicular canals [8].
Axilla and Toewebs: Corynebacterium
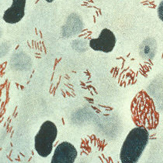
Corynebacterium is a microbe often found on the skin and is classified as a coryneform. This means that it is non-branching, not-sporing, non-acid-fast, gram-positive, nonmotile and aerobic [1].
Corynebacteria can grow at temperatures ranging from 15 to 40 degrees Celsius, but have the best growth at 37 degrees. They are also halotolerant and can grow at NaCl concentrations of up to 10 percent. Because of this, they able to live on locations of the skin with high salt content such as the axillae and in between the toes. These areas are somewhat occluded and therefore have a higher moisture and temperature. These conditions make corynebacteria more prevalent in these regions of the skin. They are found on the skin throughout the body such as the head, arms, legs and hands, but are more prevalent on the lower half [1].
They are able to use both carbohydrates and amino acids as carbon and energy sources. These macronutrients are readily available in sweat. Glucose is obtained through the hydrolysis of glycoproteins, whereas amino acids are obtained from the hydrolysis of proteins. These amino acids are required for growth. Lipophilic corynebacteria also need other nutrients which they obtain from sweat. These nutrients include riboflavin, thiamine, biotin, nicotinamide, and pantothenate [1].
Microbial Interactions of Corynebacterium and its Effects on the Dermal Environment
Corynebacteria provide the environment that allows other bacteria to grow. They are able to hydrolyze urea to NH4, which provides a nitrogen source for most cutaneous microbes. At the same time they are dependent on other microbes such as Staphylococcus and Propionibacterium. These types of bacteria provide fatty acids which they obtain from lipids. And in this way they have a complex relationship with these other organisms[1].
Corynebacterium make up 19% of microbes found on the human skin. And the frequency of detection is much higher in the month of May than in any other time of the year. This is probably due to the increase in average temperature from 8.2 to 16.5 degrees Celsius.(1) Some species of Corynebacterium, such as Corynebacterium diphtheriae and Corynebacterium ulcerans, are capable of causing skin lesions [12]. These ulcers are often co-infected with Staphylococcus aureus and group A streptococci. These infections can spread to other areas of the body causing pharyngeal infections.(1) Other species like, Corynebacterium tenuis are the cause for trichomycosis axillaris. This is a colonization of the hair shaft in sweat glands in areas such as armpits and pubic area. It consists of a tightly packed overgrowth of this microbe [13].
Hands and Face: Propriobacterium acnes
Propionibacterium acnes, named for its production of propionic acid, is a commensal gram positive bacterium, found in the sebum produced by sebaceous glands in the skin [14]. It is aerotolerant, allowing it to grow in aerobic conditions but preferring anaerobic fermentation [15]. It tends to grow on hair follicles where there are reduced levels of oxygen and in areas with aerobic organisms that consume oxygen from their immediate environment [16]. Its optimal temperature is 37*C, optimal pH 5.5-6.0. P. acnes is sensitive to UV irradiation but is increasingly antibiotic resistant [17]. It metabolizes fatty acids and sebaceous fluid secreted by the pores they reside in. The products of its fermentation of free short-chained fatty acids assist in its colonization on hair follicles. In addition, they also synthesize proteases, which kill staphylococci and others of the Propionibacteria species. The propionic and acetic acid that it produces are also seen to inhibit growth of other microbial species found in its environment. Although there is no direct benefit to our human skin in harboring such a bacterium, its existence prevents possible onset of more dangerous pathogens, as the worst pathogenic consequences it can bring about are painful lesions and disfiguring acne scars. Its presence occupies the skin such that no dangerously pathogenic microbes can do it harm [19].
Microbial Interactions of P. acnes
Little beneficial interactions are seen among skin microbes; usually, they are rather antagonistic. However, a beneficial interaction is seen with P. acnes. As one of the more predominant cutaneous microbes, its existence depends on aerobic microbes, including Corynebacterium jeikeium and Acinetobacter, and facilitative microbes, such as staphylococci and Dermabacter hominis, for these microbes consume oxygen and provide P. acnes with their ideal atmosphere to flourish. Without these aerobic and facilitative microbes, they are unable to grow in an aerobic environment. In turn, P. acnes provide other microbes like micrococci and Brevibacteria with propionic and acetic acids, used as carbon sources. Additionally, the bacteria requires vitamins for growth, including biotin, nicotinamide, pantothenate, and thiamine.
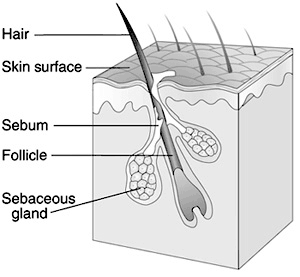
. Other examples of beneficial interactions include staphylococci producing lactate that is used as a carbon source for Actinobacteria and keratin hydrolysis that produces amino acids used by staphylococci, micrococci, and Brevibacterium [16].
Effects of P. acnes on the Dermal Environment
Although normally nonpathogenic, P. acnes paves the way for opportunistic pathogens to generate infections. Like most of the skin’s normal flora, it does not pose as a threat until the skin is compromised [19]. An accumulation of P. acnes in sebum-rich areas is implicated in the onset of acnes vulgaris, a skin condition affecting 80% of adolescents in America [20]. P. acnes can bind to oleic acid, which is a major component of sebum. Oleic acid, in turn, promotes co-aggregation, of the organism. This co-aggregation, along with the interaction of the microcolony with oleic acid, would help maintain the organism in the habitat; in this case, hair follicles. Sebum is normally excreted through pores of the skin. Acne, characterized by inflamed lesions, results when sebum and oil builds up under the skin and is further blocked by blackheads [16].
Though not a direct cause of acnes vulgaris, the presence of P. acnes certainly aids in its onset. Research shows that an acnes inflammation is deemed less severe after treatment with antibiotics that target P. acnes, although P. acnes is becoming increasingly antibiotic resistant [20]. The putative genome of P. acnes has 2333 genes encoding metabolic products including the degradative enzyme lipase that damages host tissue, as well as the heat shock protein that induces an immune response that triggers inflammation [21]. Lipases can also digest excess skin oil and sebum in regions that contain hair follicles and sebaceous glands. Blocked pores create an optimal anaerobic condition for P. acnes to thrive [14]. As P. acnes proliferate in excess, their metabolic products overwhelm the cell wall such that it is ruptured open and a bacterium, such as S. aureus, can cause irritated lesions [16].
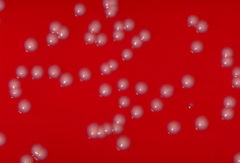
Upper Arms, Legs, and Trunk: Micrococcus luteus
Micrococcus luteus is the most common bacteria of the Micrococcus species found on the skin [22]. The bacteria mostly colonize the legs, arms, and head [24]. These areas of the body do not hold as much moisture as other areas, like the axilla, which explains how Micrococcus luteus tolerate dry conditions. They are mainly innocuous on the skin, although immunodeficiency in the host, like HIV patients, can lead to infection.
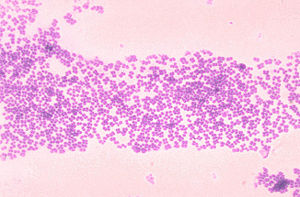
These gram-positive bacteria are tolerant at high salt concentrations. It’s halotolerant up to 7.5% NaCl, which allows it to tolerate the salinity of the skin- note that seawater is 3% NaCl [16]. Sweat and sebaceous glands greatly contribute to the salinity of the skin. Secretions of sweat from sweat glands to the skin are high in salt concentration and low in water. As the amount of sweating increases, the amount of salt reaching the skin’s surface increases too. The evaporation of water from the release of heat enables the salts to remain present on the skin [25]. As a result of these secretions, nutrients and minerals are present for halotolerant bacteria to survive on the skin.
The nutrients utilized by these bacteria are present on the skin environment. Their primary carbon energy source is derived from amino acids available on the skin after being secreted from sweat glands. These amino acids include arginine, cysteine, methionine, tyrosine, making M. luteus one of the most demanding nutritionally bacteria of the skin [26]. A secondary energy source is the lactic acid secreted from sweat glands to the skin’s surface, which can be converted to its lactate salt form by other bacteria [16].
Microbial Interactions of M. luteus
M. luteus can find carbon energy sources from the fermentative products of other dermal-colonizing bacteria. Proprionibacterial species – such as P. acnes – produce proprionate and acetate, which is one such potential source of energy; another example is lactate produced by Staphylococcus species [16]. Lactate may be a nutrient for bacteria as well as a contributor to skin pH. The lactate and lactic acid interconvert and contribute to the buffering of the skin pH balance; amphoteric amino acids also contribute to this buffer [25]. As a result, these symbiotic relationships between bacteria are beneficial for providing nutrient sources to each other, allowing many species to inhabit the skin. These bacteria also secrete products to their environment, which keep the skin niche in equilibrium. For example, glycerol is another carbon source used for metabolism in Micrococcus luteus [16]. Lipases are used to degrade oils on the skin into free fatty acids and glycerol. This alcohol generated is metabolized by M. luteus and releases an acid bi-product. Increasing acidity in the environment can contribute to the acidity of the skin pH. This acidity of the skin can be lethal to other microbes that prefer more basic environments. M. luteus therefore contributes to protecting the skin from unwanted microbes by contributing to acidity [16].
Effects of M. luteus on the Dermal Environment
With the functioning of protease enzymes, many amino acids are released into the environment. Itself along with other neighboring bacteria (like Malassezia species, Staphylococcus species, and Brevibacterium species) benefit from the break down of the proteins so it may acquire free amino acids [16]. Some of the products from the breakdown of these nutrients produce rancid odors [26]. For example, the stench of feet is produced by Micrococcus species breaking down nutrients. These smelly compounds are antibacterial to some bacterial species and can prevent other microbes from inhabiting the skin.
Non-Bacterial Inhabitants of the Skin
Genus Fungi, Malassezia globosa of Malassezia (Pityrosporum)

One type of fungi that is normally found on the skin is known as Malassezia globosa. M. globosa is a lipophilic, dimorphic yeast that is normally present on the healthy skin of humans [1]. It is most commonly found on host’s skin in tropical environments. It utilizes lipids as a source of carbon and energy, since it is not able to ferment sugars [2]. The lipids contain fatty acids, which M. globosa can use for growth [1]. Because fatty acids and lipids are required for this yeast, it prefers to colonize in areas of the skin that are rich in sebaceous glands such as the scalp, chest, face, and upper back [1]. The yeasts multiply by budding from a scar at the base of the cell, and occur as either spherical or cylindrical forms. Normal skin is mostly saprophytic yeast phase in the spherical form on trunk and oval on the scalp.
Factors like puberty, excessive sweating, warmer season, oil application, malnutrition, and steroids, help in massive growth of the fungus in diseased states. Studies show M. globosa acts as a chemotactic agent for leukocytes inducing inflammation and activation of dermatitis and folliculitis indicating irritant and non-immunogenic stimulant [5]. Although it normally occurs on the skin, it can also demonstrate an invasive etiology that can lead to skin diseases such as pityriasis versicolor, seborrhoeic dermatitis, and dandruff in addition to atopic dermatitis and folliculitis [1 and 2]. The condition known as Pityriasis Capitis, or dandruff, is an inflammatory scalp disorder disrupting cohesion on corneocytes. This is a result of toxin production and lipase activity of the yeast, which stimulates host immune response to the yeast’s antigens [6].
Current Research
1) Molecular Identification of the Malassezia Species [4]
Polymerase Chain Reaction has recently been used to distinguish between the species within the genus Malassezia. Previous techniques for identification of the Malassezia species were based on morphological biochemical, and physiological characteristics that were complex and time consuming. PCR provides a fast and simple method of analyzing the internal transcribed spacer, which varies between species of Malassezia, as a means of differentiation. In this study, four particular Malassezia species which include: M. globosa, M. furfur, M. sympodialasis, and M. restricta that were isolated from dermatitis infections. The specificity of primers were tested and each species of Malassezia had a specific pair primer. The strains of Malassezia were then put into a PCR assay. PCR was able to distinguish between species that were physiologically similar. The PCR method provides an efficient identification system of the malassezia species that can be used in routine practices.
2) Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds [22]

On the human skin bacterial infections can result when they have internal access to invade the host. It usually happens in open wounds or immunodeficient individuals. Treatment of infections has become a challenge since many bacteria evolve to acquire antibiotic resistance. A current study observes the antibiotic properties of honey which may be utilized as an effective antibiotic. Honey has been used since ancient times for healing wounds and respiratory infections, as it is both acidic and possessing high amounts of H2O2, volatiles, and other chemicals that are potentially useful for antibiotics.
The study used different strains of bacteria which potentially cause skin infection. They used honey from an apiary and from honey packaged in barrels. The honey from the apiary was not mixed with other apiaries. However, the packaged honey contained a mix of different sources of honey. The results showed that the honey from the apiaries was more effective in killing bacteria than the packaged honey, and it was believed to be the result of the fact that packaged honey is heated to unpack honeycombs, which may cause the beneficial properties of honey to be lost. One type of bacterium that was effectively targeted was Staphylococcus aureus, which could lead to breakthroughs in the development of antibiotics for future remedies of its increasingly mainstream antibiotic-resistance.
3) Molecular analysis of human forearm superficial skin bacterial biota [10]
Part of the difficulty with defining the bioforal lexicon of the human skin is that there has been little progress made beyond culture-dependent assays in the techniques needed to definitively determine the presence of specific genera of bacteria. These kinds of cultivation methods have been shown to be capable of cultivating less than one percent of all bacterial species. In this experiment by Gao et al., a relatively novel technique called 16S small subunit ribosomal (rRNA) gene surveying was used, which targeted a highly conserved region of the ribosomal genome across most bacterial species with RNA primers to collect a much more representative idea of the true bacterial population on the human skin.
Their analysis focused on samples from the superficial skin from the forearms of six healthy adult subjects, and indeed did demonstrate how truly limited the previous techniques’ data was. Their results showed that three phyla (Actinobacteria, Firmicutes, and Proteobacteria) accounted for 94.6% of all the bacterial clones identified. The results demonstrated that the interpersonal variation in specific bacterial colonization was much greater than previously anticipated, with very low levels of overlap between any of the six subjects. It is the hope of these researchers that these results will help to reveal the identities of populations of microbes that could play important roles in skin diseases.
4) A diversity profile of the human skin microbiota [11]
This experiment by Grice et al. also used the 16S small subunit ribosomal (rRNA) gene surveying technique that Gao used, but instead of analyzing samples from the skin of the forearm, analyzed the inner elbow region of five healthy adults. As this environment is significantly more moist, acidic, and occluded than the forearm used by Gao’s team, it was expected that a different bacterial lexicon would be observed. An additional difference was that besides swabbing the most superficial surface of the skin, this group also analyzed samples from skin scrapings and puncture biopsies, to see if the bacterial populations differed with more invasive skin sample analyses.
The results revealed that the bacterial populations differed little between the superficial swabbing and invasive puncturing samples. This was thought to be a result of the eventual exportation to the surface of all internal bacteria as new skin cells were generated, and not necessarily revealing of any actual consistency in bioflora colonization throughout the epidermis. Similarly to the Gao experiments, Grice’s investigation revealed that a small number of species dominated the skin’s landscapes, with the Proteobacteria pseudomonas species alone representing 59% of the 16S RNA sequences identified in this survey alone. She infers from the conserved nature of this consistency across the individuals that a diseased state could be the product of some alteration in the proportion of these microbes or the introduction of some novel microbe.
5) Vitamin D3 Provides Skin With Protection From Harmful Microbes [28]
In a study led by Dr. Richard Gallo of UCSD School of Medicine, Division of Dermatology, Vitamin D3 is found to be essential as a skin injury heals, protecting a wound from infection and initiating skin repair. When a body lacks or has an insufficient amount of active Vitamin D3, it becomes more vulnerable to microbe infection. The natural immune response when a body is afflicted by an abrasion is to produce an antimicrobial peptide called cathelicidin, which is needed to fight infections. Receptors on white blood cells trigger production of cathelicidin when they sense an invading bacterium. Gallo and his lab have also discovered that skin cells called keratinocytes are stimulated upon injury. They surround a wound and increases Vitamin D3 production. Vitamin D3 aids in expression of genes that identify microbes, such as CD14 and TLR2. These genes form a complex with Vitamin D3, 1,25D3, which triggers more cathelicidin production. In studying mice and human subjects, a decrease in cathelicidin led to an increase in developing infections. 1,25D3 is found to vary between races. For instance, African Americans are found to have a lower concentration of 1,25D3, thus rendering them more susceptible to infection. This may be caused by their body’s inefficient absorption of Vitamin D from sunlight.
This study is ongoing to determine if levels of Vitamin D3 are related to enhanced innate immune defenses. Clinical trials conducted observe both oral and topical Vitamin D in healthy volunteers and patients suffering from antimicrobial peptide production disorders, such as acne and eczema.
6) Newer skin treatments have shown successful prevention of Acne vulgaris [29]
Acne vulgaris is a common skin disease that has many causes. Genetics, hormonal balance, increase in size or excessive activity of the sebaceous gland, cell build up, and bacterial infections. When many of these factors come together, the first stage of acne is formed as microcomedones. When anaerobic conditions are present in microcomedones, P. acnes can overgrow and lead to papules and pustules visible on the skin. Acne has become a problem for many individuals and current skin therapies try to target the factors that cause this disease.
Analysis of current acne treatments have shown successful results. Photodynamic therapy has shown success on anaerobic bacteria. A blue light is used to target bacterial cells, and based on a specific absorption of light it induces P. acnes to produce porphyrin. As a result P.acnes die from this therapy, because this photodynamic reaction creates oxygen presence around the cells. Clinical studies have shown that this technique is effective. Based on such successful trials, this technique will be useful in the future to prevent acne. Cryotherapy uses liquid nitrogen to freeze the skin. The cold is tested to exfoliate and shed parts of the sebaceous gland. As a result less activity of the sebaceous gland is shown, so it reconstructs the skin into a calmer environment. Chemical peels have also been tested to be successful therapies. Chemicals that enhance keratolytic activity are used to restore skin balance. Chemicals like phenol are also used, which have antiseptic properties.
Overall these new therapies have shown successful reduction of acne and scarring. What must be considered in the future is the safety of these procedures. Chemical peels should be regulated to make sure the antiseptic compounds do not harm the skin. Observing the penetration of light to the skin should also be examined in photodynamic therapy for patients. Long term trials will be needed to see if these treatments last and to observe negative developing effects.
Closing Thoughts
The skin serves as the human body's most important epithelial interface between the external world of microbial threats and its internal environment. As the body's largest organ, the skin serves to not only physically protect the bloodstream, but also helps with thermoregulation and osmoregulation. Though the skin has evolved to establish itself as a relatively toxic environment for most bacteria to land upon, there are a considerable number of bacteria that have similarly evolved in ways that make them capable of colonizing the nutrient-rich surface that the skin presents. The nutrients and conditions available are not universally consistent throughout the skin; however, as the moisture, pH, temperature, and occlusion varies and creates different niches for different bacteria in different regions of the body. The three specific niches identified and explored here were the moist and acidic axilla and toewebs; the dry and basic upper arms, legs, and trunk; and the intermediate hands and face. The predominant bacteria capable of colonizing these three regions -- as contemporary science is capable of identifying them, at least -- all perform some commensalist or symbiotic function for their host, while benefiting from the ecological niche they are provided with for their own subsistence.
References
1. Wilson, Michael. Bacteriology of Humans: an Ecological Perspective. Blackwell Publishing, 2008.
2. Wilson, Michael. Microbial inhabitants of Humans: Their Ecology and Role in Health and Disease. Cambridge University Press, 2005.
5. Inamadar AC, Palit A. "The genus Malassezia and human disease." Indian J Dermatol Venereol Leprol [serial online] 2003 [cited 2008 Aug 26];69:265-70. Available from: http://www.ijdvl.com/text.asp?2003/69/4/265/4990
8. Aly, Raza. Clinical Skin Microbiology. Springfield, IL: Thomas Books, 1987. 11-35.
9. Elias, Peter M., and Kenneth R. Feingold, eds. Skin Barrier. Danbury: Marcel Dekker Incorporated, 2006.
13.O'Dell ML (1998). "Skin and wound infections: an overview". Am Fam Physician 57 (10): 2424–32.
14.Propionibacterium Acnes and Hydrogen Peroxide. Acne Talks. <http://www.acnetalks.com/pimple/Acne-Treatment/Methods/Propionibacterium-Acnes-And-Hydrogen-Peroxide.htm>. Accessed 27 August 2008.
15.Propionibacterium acnes KPA171202 project at Goettingen Genomics Library. NCBI Entrez Genome Project. < http://www.ncbi.nlm.nih.gov/sites/entrez?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=12460>. Accessed 27 August 2008.
16. Wilson, Michael. Microbial Inhabitants of Human: Their Ecology and Role in Health and Disease. Cambridge University Press, 2005.
17. Ingham, Eileen The Immunology of Propionibacterium acnes and Acne. Current Opinion in Infectious Diseases. 1999. Vol. 12(3): p. 191-197.
18. Prater, Alicia Mae. Not All Bacteria Are Bad: Digestion and Immunity Are Aided By Microbes. Suite101, 8 May 2008. < http://bacteriology.suite101.com/article.cfm/not_all_bacteria_are_bad>. Accessed 24 August 2008.
19. Oliver, David. Microbes and You: Normal Flora. The Science Creative Quarterly, Issue Three, Sept07-Apr08. < http://www.scq.ubc.ca/microbes-and-you-normal-flora/>. Accessed on 24 August 2008.
20. Ingram, Eileen, et. al. Inflammation of Acne vulgaris: Failure of Skin Micro-organisms to Modulate Keratinocyte Interleukin 1α Production in vitro. Dermatology 1998; 196:86-88.
21. Brüggemann, Holger et al. The Complete Genome Sequence of Propionibacterium Acnes, a Commensal of Human Skin. Science. 2005. 305: p. 671-672.
22. Basualdo, C., Sgroy, V., Finola, M.S., Marioli, J.M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Veterinary Microbiology. (2007) v. 124, pgs 375-381
23. Clark, D.J., Hawrylik, S.J., Kavanagh, E., and Opheim D.J. Purification and Characterization of a Unique Alkaline Elastase from Micrococcus luteus. Protein Expression and Purification. (2000) v. 18, pgs 46-55
24. Kloos*, W.E., and Musselwhite M.S. Distribution and Persistence of Staphylococcus and Micrococcus Species and Other Aerobic Bacteria on Human Species. Applied Microbiology. (1975) v. 30, pg. 381-395
25. Marples, M. J. The Ecology of the Human Skin. Charles C Thomas Publisher. Springfield, Ill. (1965) pgs103-154
26. Noble, W.C. The Skin Microflora and Microbial Skin Disease. Cambridge University Press. New York, NY.(1993) pgs 1-70
27. Slonczewski, J.L., and Foster, J.W. Microbiology, An Evolving Science. W.W. Norton & Company, Inc. New York, NY. (2009) pg 877
28. Schauber, J. and Gallo, R.L. “Antimicrobial peptides and the skin immune defense system.” Journal of Allergy and Clinical Immunology (2008) v 122, pgs 261-266
29. Kaszuba A., Bartkowiak R., and Kaszuba A. “Adjunct and relating to a procedure methods of the management of acne vulgaris and traces after treatment” Aesthetic Dermatology (2006) v. 8, pgs 9-13
Edited by Patrick A. McGhee, Susan Lin, Eric Pham, Pavithra Ramasubramanian, Deeba Pourmand, Wei Chien, Leo Borjon, David Juma, Cynthia Wong, students of Rachel Larsen
