Skin
General Introduction
The skin is an organ of several different epithelial systems that serves multiple purposes as the largest interface between the host’s internal environment and the external world [1,2]. Composed of an inner dermis and outer epithelium, it has a surface area generally in the range of 1.75 square meters 5 kilograms, and is considered to be the largest organ of the human body [1,2]. The variation in environmental conditions such as moisture, carbohydrate content, temperature, and pH observed between different regions of the skin contributes directly to the bacterial bioflora observed there upon. The driest areas are the upper arms and legs, the most moist are the axilla and toewebs. The areas of the face and hands are considered a distinct category unto themselves that falls in between the dry and moist extremes [2]. The skin serves a number of key functions, with its most basic purposes surrounding its role as a physical barrier protecting the host's internal environment from external pathogens and osmoregulation.
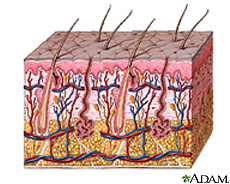
DESCRIPTION OF THE SKIN
Location and Significance
The skin’s key role as the physical barrier delineating between the bloodstream and external environment carries many responsibilities. The dermal epithelium is charged with preventing excess water loss, controlling and maintaining its own plasticity and elasticity, and protecting against UV radiation; but also must integrate all these duties in such a way that also provides a barrier against the natural microbial proclivity for seeking out and colonizing in the skin’s nutrient-rich environment. (Elias, Feingold, 363?)
While there is some consistency in the microbial biodiversity between all regions of skin, generally speaking there are three regions of skin that differ in their specific bacterial flora. These regions are (1) axilla and toewebs; (2) hands, face, and trunk; and (3) upper arms and legs. These anatomical distinctions in microbial biodiversity are products of their physical conditions, most importantly their differences in pH, moisture, body temperature, and concentrations of skin lipids.
Skin epithelium functions to keep microbes off the rest if the body by:
- Acting as a physical barrier against microbe penetration to tissues underneath [2]
- Secreting a mucus layer so that microbes can not permanently attach to the epithelial cells beneath [2]
- Shedding or keratinization of the outermost skin cells so microbes are removed from the body [2]
- Secreting antimicrobial peptides and proteins to kill off microbes or at least prevent their growth [2].
Physical Conditions of the Skin
The composition of the skin’s most superficial layers is such to optimize its prevention of bacterial growth on its surface. Examples of this can be found in its temperature, low carbohydrate and water content, acidic pH, and antimicrobial peptide activities.
The acidic quality of the skin plays an important role in combating bacterial growth. The skin’s pH generally fluctuates between 5.6 and 6.4 depending on the region of the body it is covering, establishing an environment that few species of bacteria are capable of proliferating in. The skin’s acidity results from lactic acid from the host cells and the microbes, the amino acids from sweat, the fatty acids from sebum, and acids produced during keratinization [1]. Although the skin is acidic, it is only suitable for neutrophile growth and not acidophiles or alkaliphiles[1].
The temperature of the skin varies depending on the location on the body. Toes and fingers tend to have the lowest temperatures, while the axillae and the groin tend to have the highest [1]. The temperatures are usually 25-35 degrees Celsius, which is ideal for mesophiles rather than thermophiles or psychrophiles [1]. The temperature only varies slightly so it there is not a dramatic selection for microbes colonizing certain areas, but does affect the growth rate of the microbes present [1].
The moisture content on the skin is generally low, which limits the survival and growth of microbes. However, the eccrine glands can produce sweat, which increases the moisture on the surface of the skin; especially in areas where evaporation does not occur easily, such as the toes and axillae [1]. Microbes tend to have greater populations in those occluded areas since there is an accumulation of secretions[1].
The skin generally has a high oxygen concentration, so it acts primarily as an aerobic environment for anaerobes to grow [1]. However, the hair follicles inside the skin provide a microaerophillic and/or anaerobic environment that have lower levels of oxygen, so that microaerophiles and obligate anaerobes can also grow [1].
Influence by Adjacent Communities
The skin is always exposed to the external environment. Ecological factors such as climate and habitat have great influence on the microbial flora that colonize the skin. Generally speaking, gram-negative bacilli more frequently colonize regions of the skin with higher moisture content or partial occlusion. Contact with dirt, for example, can introduce non-indigenous or harmful microbes onto the skin. Even air can influence the microbe communities by preventing the airborne microbes from settling on the skin [2]. The openings of the host’s body such as the nose, mouth, urethra, and rectum can also introduce microbes from those regions on to the skin [2].
Conditions Causing Environment Changes
Do any of the physical conditions change? Are there chemicals, other organisms, nutrients, etc. that might change the community of your niche.
Variations in the amount or concentration of sebaceous and sudoriferous glands can affect the skin's temperature, water content, concentration nutrients, osmolarity, pH, and concentration of antimicrobial substances [2]. This is because sebaceous glands are major sources of nutrients for microbes, while the sudoriferous glands produce sweat as a source of water on the skin [2]. Both glands also produce antimicrobial substances important to the skin [2].
Clothing, air-conditioning, or housing, for example, can be considered forms of protection against extreme environments. However, the conjunctiva and exposed regions of the skin have greater fluctuations in temp, humidity, and so forth, in comparison to other bodily systems [1]. Covering areas of the skin, for instance, can prevent the evaporation of water and encourage a build up of secretions and alter the pH [2].
Even host characteristics such as age, gender, host’s nutrition, stress, emotional state, disability, hospitalization, personal hygiene, lifestyle, occupation, living conditions, and so on can affect the skin environment[1].
INHABITANTS OF THE THREE CATEGORICAL REGIONS OF SKIN
Microbes are described as “nonpathogenic” if they demonstrate limited growth on the epithelial surface, while those that can invade and have the potential to reach levels of unrestrained growth are defined as “pathogenic” (Elias, Feingold, 363?). The majority of the microorganisms that live in the epidermis live in its most superficial layer, known as the stratum corneum, while there are certain species of bacteria that reside in the more secluded areas of follicular canals.
Axilla and Toewebs: Corynebacterium
Microbes Present
Microbial Interactions
Microbial Effects on the Environment
Hands and Face: Propriobacterium acnes
Microbes Present
Microbial Interactions
Microbial Effects on the Environment
Upper Arms, Legs, and Trunk: Micrococcus luteus
Microbes Present
Microbial Interactions
Microbial Effects on the Environment
Other Organisms Present
Microbes Present
Microbial Interactions
Microbial Effects on the Environment
Other Organisms Present
Genus Fungi, Malassezia globosa of Malassezia (Pityrosporum)
One type of fungi that is normally found on the skin is known as Malassezia globosa. It is one species belonging to the genus Malassezia. It is a lipophilic, dimorphic yeast that is normally present on the healthy skin of humans [1]. It is most commonly found on host’s skin in tropical environments. It utilizes lipids as a source of carbon and energy, since it is not able to ferment sugars [2]. The lipids contain fatty acids, which Mal. globosa can use for growth [1]. Because fatty acids and lipids are required for this yeast, it prefers to colonize in areas of the skin that are rich in sebaceous glands such as the scalp, chest, face, and upper back [1]. The yeasts multiply by budding of a scar at the base of the cell, and occurring as either spherical or cylindrical forms. Normal skin is mostly saprophytic yeast phase in the spherical form on trunk and oval on the scalp. The diseased parasitic mycelial forms occur in short septates of filamentous cell with some branching. Factors like puberty, excessive sweating, warmer season, oil application, malnutrition, and steroids, help in massive growth of the fungus in diseased states. Studies show Malassezia acts as a chemotactic agent for leukocytes inducing inflammation and activation of dermatitis and folliculitis indicating irritant and non-immunogenic stimulant [5]. Although Mal. globosa occurs normally on the skin, it can also play a pathogenic role, which is why it has been associated with skin diseases such as pityriasis versicolor, seborrhoeic dermatitis, and dandruff in addition to atopic dermatitis and folliculitis [1 and 2]. For instance,Pityriasis Capitis, also known as dandruff, is an inflammatory scalp disorder disrupting cohesion on corneocytes. This is a result of toxin production and lipase activity of the yeast, which stimulates host immune response to the yeast’s antigens [6].
Microbial Interactions
Microbe-microbe interactions on the skin tend to either be beneficial or antagonistic.
- Beneficial interactions between microbes, whether it is commensalism or synerism, exist between many microbes on the skin.
The nutritional web of interactions between the microbes is shown to be simple and it is easy to understand the benefits of other microbes in the niche. For instance, propionibacteria is shown to excrete acids that can be used by micrococci, Acinetobacter spp., and brevibacteria as a source of carbon and energy. Lactate produced by staphylococci can be used as a carbon and energy source for Acinetobacter spp and micrococci as well.
In addition to providing nutritional benefits , interacting microbes can also provide hospitable environments for other microbes. This type of commensalism is shown by Malassezia spp., which metabolizes lauric acid that is toxic to propionibacterim acnes, so that the propionibacterim acnes can survive where lauric acid is plentiful [1].
- Antagonistic substances may be produced by cutaneous microbes for competition against non-indigenous microbes.
- CO2 is produced by many bacteria against dermophyte growth [1]
- Lysozymes are produced by staphylococci which kills micrococci, Brevibacterium spp., and Corynebacterium spp. [1].
- Proteases are produced by P. acnes to kill off other Propionibacterium spp. and staphylococci[1].
- Propionic acid is produced by propionibacteria, which inhibits other species from growing at low pH’s on the skin [1].
- Acetic acid is produced by propionibacteria and Dermabacter hominis to inhibit the growth of other species [1].
- Lactic acid is produced by staphylococci and D. hominis to inhibit the growth of other species [1].
- Bacteriocins are produced by staphylococci, Coryebacterium spp., Propionibacterium spp., Micrococcus spp., and Brevibacterium spp. to inhibit the growth of or kill cutaneous organisms [1].
- Antagonistic substances may be produced by cutaneous microbes for competition against non-indigenous microbes.
Do the microbes change their environment?
Do they alter pH, attach to surfaces, secrete anything, etc. etc.
Microbe Metabolism Affecting the Environment
Do they ferment sugars to produce acid, break down large molecules, fix nitrogen, etc. etc.
- Mal. globosa produces proteases that can break down skin proteins, so that amino acids become available[1]. It can also take more of a pathogenic role by producing toxic metabolites or hydrolases that degrade sebum, freeing fatty acids that came from sebaceous triglycerides, consuming certain saturated fatty acids, and leaving behind the unsaturated fatty acids on the skin which can cause irritation, inflammation, and flaking of the scalp [3].
- Corynebacterium spp., staphylococci, and Brevibacterium spp. produce ureases which can break down urea into ammonium ions as a nitrogen source, which is sued by most cutaneous organisms[1 and 2].
Current Research
Enter summaries of the most recent research. You may find it more appropriate to include this as a subsection under several of your other sections rather than separately here at the end. You should include at least FOUR topics of research and summarize each in terms of the question being asked, the results so far, and the topics for future study. (more will be expected from larger groups than from smaller groups)
Molecular Identification of the Malassezia Species (2008)
Polymerase Chain Reaction has recently been used to distinguish between the species within the genus Malassezia. Previous techniques for identification of the Malassezia species were based on morphological biochemical, and physiological characteristics that were complex and time consuming. PCR provides a fast and simple method of analyzing the internal transcribed spacer, which varies between species of Malassezia, as a means of differentiation. In this study, four particular Malassezia species which include: Mal. globosa, Mal. furfur, Mal. sympodialasis, and Mal. restricta that were isolated from dermatitis infections. The specificity of primers were tested and each species of Malassezia had a specific pair primer. The strains of Malassezia were then put into a PCR assay. PCR was able to distinguish between species that were physiologically similar.. The PCR method provides an efficient identification system of the malassezia species that can be used in routine practices [4].
"A diversity profile of the human skin microbiota" (2008)
By surveying different depths of skin: swab, scrape, and punch biopsy, the skin was tested for the different skin microbiota. Similarities between the human and mouse were found to be the strongest. The human inner elbow was compared to the mouse ear skin, resulting in similarities. Test was done with the help of Polymerase Chain Reaction and the test of 16SrRna (small subunit ribosomal genes- site specific phylogenic relationships). This study of healthy human skin microbiota can help determine the complex physiological interactions between the skin and the microbes that inhabit this environment [7].
References
1. Wilson, Michael. Bacteriology of Humans: an Ecological Perspective. Blackwell Publishing, 2008.
2. Wilson, Michael. Microbial inhabitants of Humans: Their Ecology and Role in Health and Disease. Cambridge University Press, 2005.
5. Inamadar AC, Palit A. "The genus Malassezia and human disease." Indian J Dermatol Venereol Leprol [serial online] 2003 [cited 2008 Aug 26];69:265-70. Available from: http://www.ijdvl.com/text.asp?2003/69/4/265/4990
Edited by Patrick A. McGhee, Susan Lin, Eric Pham, Pavithra Ramasubramanian, Deeba Pourmand, ____________________________________, students of Rachel Larsen
