Stenotrophomonas rhizophila: Difference between revisions
| (5 intermediate revisions by 2 users not shown) | |||
| Line 22: | Line 22: | ||
==Description and Significance== | ==Description and Significance== | ||
''Stenotrophomonas rhizophila'' is a Gram negative bacilli[[#References|[1]]]. ''S. rhizophila'' can be found within a range of host-associated locations from stems, leaves, or the rhizosphere. In tomatoes, for instance, it is more common to find ''S. rhizophila'' within its leaves [#References|[12]]]. In cotton or sweet pepper, there will be a higher density of ''S. rhizophila'' in its rhizosphere and begin to lower as you go up the plant. For potato tubers, colonization of the endosphere was found to be more common.[[#References|[5]]] | ''Stenotrophomonas rhizophila'' is a Gram negative bacilli[[#References|[1]]]. ''S. rhizophila'' can be found within a range of host-associated locations from stems, leaves, or the rhizosphere. In tomatoes, for instance, it is more common to find ''S. rhizophila'' within its leaves [[#References|[12]]]. In cotton or sweet pepper, there will be a higher density of ''S. rhizophila'' in its rhizosphere and begin to lower as you go up the plant. For potato tubers, colonization of the endosphere was found to be more common.[[#References|[5]]] | ||
Recent agricultural practices have resulted in salinized soils increasing the likelihood of plant life being affected by soil borne diseases. Fortunately the production of osmoprotective substances, trehalose and glucosylglycerol, has allowed for plant-bacteria symbiotic relations to form where the cultivation of biofilms enables plants to tolerate osmotic pressures by fighting off deleterious and pathogenic rhizosphere microorganisms [[#References|[5]]]. For example, in Uzbekistan’s highly salinized soils the presence of ''S. rhizophila'' dramatically increased plant growth by 180%.[[#References|[4]]] | Recent agricultural practices have resulted in salinized soils increasing the likelihood of plant life being affected by soil borne diseases. Fortunately the production of osmoprotective substances, trehalose and glucosylglycerol, has allowed for plant-bacteria symbiotic relations to form where the cultivation of biofilms enables plants to tolerate osmotic pressures by fighting off deleterious and pathogenic rhizosphere microorganisms [[#References|[5]]]. For example, in Uzbekistan’s highly salinized soils the presence of ''S. rhizophila'' dramatically increased plant growth by 180%.[[#References|[4]]] | ||
| Line 34: | Line 34: | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
''S. rhizophila'' may form a monospecies biofilm, but is more likely to form a multispecies biofilm due to synergistic interactions leading to an increased biomass[[#References|[7]]]. | ''S. rhizophila'' may form a monospecies biofilm, but is more likely to form a multispecies biofilm due to synergistic interactions leading to an increased biomass[[#References|[7]]]. | ||
''S. rhizophila'' is a chemoorganoheterotroph and can subsist off of a variety of organic products. | ''S. rhizophila'' is a chemoorganoheterotroph and can subsist off of a variety of organic products. ''S. rhizophila'' is able to oxidize a significant element of plant roots, xylose. Along with xylose, other compounds that can be oxidized consist of D-trehalose, succinamic acid, DL-lactic acid and many others [[#References|[12]]]. ''S. rhizophila'' also participates in degradation of lignocellulose, though it can not directly degrade cellulose as it does not produce B-glucosidase[[#References|[11]]]. | ||
''S. rhizophila'' has important molecular products which help in its symbiosis with plants. ''S. rhizophila'' produces spermidine which has been shown to increase plant growth as well as provide a molecular base for other polyamines that can protect against drought and salinity[[#References|[2]]]. ''S. rhizophila'' also excretes glucosylglycerol(GG) and trehalose, which have high water retaining capabilities, into soil as salinity increases. This further increases plants' resistance to salinity as GG helps promote cell division and growth, despite conditions that would favor cell shrinkage[[#References|[3]]]. In addition, ''S. rhizophila'' produces dodecanal which inhibits fungal mycelial growth as well as chitinases which degrade fungal cell walls[[#References|[6]]]. | ''S. rhizophila'' has important molecular products which help in its symbiosis with plants. ''S. rhizophila'' produces spermidine which has been shown to increase plant growth as well as provide a molecular base for other polyamines that can protect against drought and salinity[[#References|[2]]]. ''S. rhizophila'' also excretes glucosylglycerol(GG) and trehalose, which have high water retaining capabilities, into soil as salinity increases. This further increases plants' resistance to salinity as GG helps promote cell division and growth, despite conditions that would favor cell shrinkage[[#References|[3]]]. In addition, ''S. rhizophila'' produces dodecanal which inhibits fungal mycelial growth as well as chitinases which degrade fungal cell walls[[#References|[6]]]. | ||
Latest revision as of 05:21, 24 April 2017
Classification
Domain: Bacteria
Phylum: Proteobacteria
Class: Gammaproteobacteria
Order: Xanthomonadales
Family: Xanthomonadaceae
Species
|
NCBI: Taxonomy |
Stenotrophomonas rhizophila
Description and Significance
Stenotrophomonas rhizophila is a Gram negative bacilli[1]. S. rhizophila can be found within a range of host-associated locations from stems, leaves, or the rhizosphere. In tomatoes, for instance, it is more common to find S. rhizophila within its leaves [12]. In cotton or sweet pepper, there will be a higher density of S. rhizophila in its rhizosphere and begin to lower as you go up the plant. For potato tubers, colonization of the endosphere was found to be more common.[5]
Recent agricultural practices have resulted in salinized soils increasing the likelihood of plant life being affected by soil borne diseases. Fortunately the production of osmoprotective substances, trehalose and glucosylglycerol, has allowed for plant-bacteria symbiotic relations to form where the cultivation of biofilms enables plants to tolerate osmotic pressures by fighting off deleterious and pathogenic rhizosphere microorganisms [5]. For example, in Uzbekistan’s highly salinized soils the presence of S. rhizophila dramatically increased plant growth by 180%.[4]
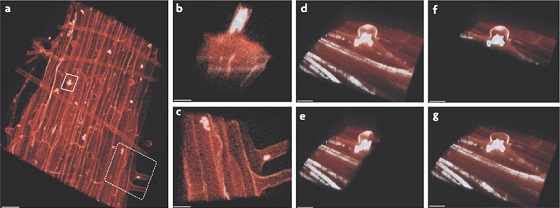
Colonies of S. rhizophila in tomato plant roots[6]
Genome Structure
Stenotrophomonas rhizophila has a single circular genome with a length of 4,648,976 base pairs[1]. It shares a high degree of sequence similarities among members of the Stenotrophomonas genus. All members of the genus share genes for host invasion, antibiotic resistance, and anti-fungal properties. While these genes would normally be present in pathogens, S. rhizophila maintains non-pathogenicity due to its loss of virulence factors and heat shock factors. Instead, S. rhizophila maintains genes for spermidine, plant cell-wall degrading enzymes, and high salinity resistance. S. rhizophila also maintains a suite of genes needed for forming biofilms, such as flagella production, surface polysaccharides, and adhesion.[2]
Cell Structure, Metabolism and Life Cycle
S. rhizophila may form a monospecies biofilm, but is more likely to form a multispecies biofilm due to synergistic interactions leading to an increased biomass[7].
S. rhizophila is a chemoorganoheterotroph and can subsist off of a variety of organic products. S. rhizophila is able to oxidize a significant element of plant roots, xylose. Along with xylose, other compounds that can be oxidized consist of D-trehalose, succinamic acid, DL-lactic acid and many others [12]. S. rhizophila also participates in degradation of lignocellulose, though it can not directly degrade cellulose as it does not produce B-glucosidase[11].
S. rhizophila has important molecular products which help in its symbiosis with plants. S. rhizophila produces spermidine which has been shown to increase plant growth as well as provide a molecular base for other polyamines that can protect against drought and salinity[2]. S. rhizophila also excretes glucosylglycerol(GG) and trehalose, which have high water retaining capabilities, into soil as salinity increases. This further increases plants' resistance to salinity as GG helps promote cell division and growth, despite conditions that would favor cell shrinkage[3]. In addition, S. rhizophila produces dodecanal which inhibits fungal mycelial growth as well as chitinases which degrade fungal cell walls[6].
Ecology and Pathogenesis
Stenotrophomonas rhizophila is an active participant in the rhizosphere, as well as endosphere, and can be found in all of plant phylogeny. S. rhizophila produces osmoprotective substances and provides plants with defenses from bacterial and fungal pathogens[10]. S. rhizophila also forms the core with a few other soil bacteria which participates in the degradation of lignocellulose[11].
With the potential to colonize plants' outer surface and S. rhizophila’s close relation to Stenotrophomonas maltophilia, there have been raised concerns over harvesting products whose surface has been colonized [5]. However, S. rhizophila does not carry the pathogenic traits essential to S. maltophilia's harm to humans and is incapable of growing in human body temperatures as it only grows below 37 °C [9]. Since bacteria that participate in biofilms enjoy increased lateral gene transfer[8], there is growing concern that the addition of a few new genes could induce a potent pathogen.[2]
References
[1] NCBI "Stenotrophomonas rhizophila". NCBI Genome Assembly. 2013. Web. 21 Apr 2017.
Author
Page authored by Esmeralda Martinez and Micah Maassen, students of Prof. Jay Lennon at Indiana University.