Stomach
Who am I?
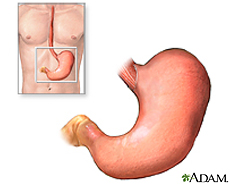
The stomach is an organ located between the esophagus and the small intestine. Food enters through the head of the stomach (cardia) and exits through the tail (pylorus) after digestion. The volume of the stomach increases at meal times and decreases as chyme leaves the stomach and enters the small intestine. The relaxed (empty) stomach has folds of lumen, which expands upon food arrival and almost disappears in a full stomach [10]. Enzymes and acids are secreted in the stomach to break down food molecules, which is stored for gradual energy use.
Animals have one of three main types of diets: Omnivores, Carnivores, or Herbivores. More specifically, human, cat, and cow respectively represent these diets. These three species are categorized by the nutrients that they consume and are distinguished by the unique characteristics of their stomach adapting to these different nutrients [11].
Omnivores: Human
special enviroment
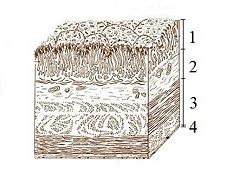
The lumen of the stomach has four distinct layers: 1. Mucosa: an epithelial layer that lines the lumen; 2. Submucosa: a matrix of connective tissue that contains blood vessels and nerves; 3. Muscularis: consists mainly of smooth muscle tissue; 4. Serosa: a thin layer of connective and epithelial tissue external 0.2 mm to the muscularis.
The stomach has three specialized cells that are located in the lining of numerous deep pits in the stomach wall. The mucus cells secrete mucus that lubricates the stomach cells, which provides protection from the stomach’s acidic environment. The chief cell secretes an enzyme known as Pepsinogen, which is an inactive form of pepsin that helps to denature proteins from absorbed nutrients inside the stomach. Lastly, the parietal cell secretes hydrochloric acid, which it is responsible for the stomach’s acidic environment [reference].
Microbes
Bacteria living in human stomach are:
Rhinovirus Tropheryma whipplei
*Most of the bacteria dies as they enter the human stomach due to low pH. However, Helicobacter pylori can colonize in the stomach.
Helicobacter pylori
- Classification
Bacteria(Domain); Proteobacteria (Phylum); Epsilon Proteobacteria (Class); Campylobacterales (Order); Helicobacteraceae (Family); Helicobacter (Genus); pylori (Species).
- Significant Discovery of H. pylori
The discovery of Helicobacter pylori as an infectious agent responsible for peptic ulcer disease marked a turning point in our understanding of gastrointestinal microbial ecology and disease. The accepted medical paradigm about stomach ulcers believed that no bacterium can live in human stomach due to low pH environment. So, they believed that the excess acid causes stomach ulcer by damaging the gastric mucosa, and treatment should be aimed at reducing or neutralizing that acid. However, in 1982, Barry Marshall and J. Robin Warren isolated a new bacterium and showed the relationship between the bacterium and diseases such as gastritis and stomach ulcers [4]. Marshall and Warren cultured the new bacterium and soon noticed that the new organism was similar to Campylobacter in several respects, including curved rod-shaped morphology, optimal growth under microaerophilic conditions, failure to ferment glucose, and G+C content of 34%. The Campylobacter-like organism was first referred to as “pyloric Campylobacter” and in 1985, it was validated as Campylobacter pyloridis. In 1987, it was again revised to Campylobacter pylori. Even though C. pylori resembled Campylobacter in many aspects, there were some distinct features: flagellum morphology, fatty acid content, and 16S rRNA sequence. Therefore, C. pylori was transferred to a new genus, Helicobacter, and renamed Helicobacter pylori in 1989. H. pylori was the first member of the new genus and the genus Helicobacter was expanded tremendously and new species are regularly included. The majority of these new Helicobacter species are found in the stomachs and intestines of different animals. [2, 3] Although isolating H. pylori was a significant work, they still did not prove whether the bacteria were the cause of the inflammation with which they were associated or whether they occurred as a result of it. Using Koch’s postulates, Marshall confirmed the connection between H. pylori and gastritis, but since stomach ulcer did not occur, that connection was still unproven. Eventually, the connection between H. pylori and ulcers was deduced from epidemiological studies showed an increased incidence of ulcers in persons infected with the bacteria [4].
- Morphology, Growth Conditions, and Special Features
H. pylori is an S-shaped bacterium with 1 to 3 turns, 0.5 X 5 μm in length, with a tuft of 5 to 7 polar sheathed flagella in one end. The cell is a gram-negative bacterium consisting outer and inner membranes that are separated by the periplasm [1]. Its morphology is similar to C. jejuni; it was initially named “pyloric Campylobacter” for this reason [6]. The nucleoid material and ribosomes exist in the dense cytoplasm and an electron-lucent area is located in the terminal regions. Associated with this region and located near the flagella insertion site is a “polar membrane.” This is an additional electron-dense band 6 to 8 nm thick located 20nm below the plasmic membrane yet linked to it. ATPase molecules are probably located at this site to generate energy for motility or cell wall synthesis [1].
H. pylori is usually located within the thick mucous layer in close proximity to gastric epithelial cells, which is an acid environment where most bacteria normally can’t survive [1]. It also typically grows under microaerobic conditions at 37°C which is close to human body temperature [3]. A weakening of the mucous barrier by H. pylori, leading in some cases to its collapse, has been proposed as H. pylori possesses a gene that is almost identical to a mucinase gene of Vibrio cholerae. Such mucinase activity may be responsible for the dissolution of the net-like structure of the mucus and the variously sized cave-like structure of the mucus and the variously sized cave-like clear areas surrounding H. pylori as observed in vivo with electron microscopic techniques. However, studies in vitro suggest that the loss of gel structure might also arise from high local pH generated by the urease activity of H. pylori rather than by mucolytic activity. Furthermore, H. pylori can inhibit the secretory response of mucous cells in vitro, indicating a potential deleterious effect on the quantity of this primary defense mechanism of the gastric mucosa [1].
- Colonization of H. pylori in stomach
Urease expression and motility by flagella permit H. pylori to survive transiently in an acid environment and to colonize persistently the mucous layer [1]. There are three essential factors for H. pylori to colonize the gastric mucosa: flagella, urease, and adhesions [2]. Firstly, H. pylori’s unique flagella in one end and its curved morphology cause screw-like movements, which may enable the organism to penetrate the mucin layer. The motility of H. pylori is increased when the viscosity of the media is increased in vitro and transverses a methyl glucose solution 10 times more efficiently than Escherichia coli, but the motility is pH dependent and impaired at a pH below 4 [2]. Its flagella are composed of flagellin and are surrounded by a membranous sheath containing LPS and protein generating an immune response. H. pylori-mediated reduction of mucus synthesis or secretion may also assist the bacterium in gaining access to the epithelium and persisting at this location [7]. Secondly, urease is one of the most necessary enzymes in H. pylori pathogenesis because it maintains a pH-neutral microenvironment around the bacteria which is necessary for survival in the acidic stomach. It is well established that H. pylori grows best at neutral pH and fails to survive at a pH below 4.0 or above 8.2 in the absence of urea. Urease converts urea, of which there is an abundant supply in the stomach (from saliva and gastric juices), into bicarbonate and ammonia, which are strong bases. This creates a cloud of acid neutralizing chemicals around the H. pylori, protecting it from the acid in the stomach. [2, 5] Lastly, H. pylori adheres to mucin and binds specifically to gastric mucosa epithelial cells both in vivo and in vitro [2]. The adherence allows the bacteria to anchor themselves to the epithelial layer, but bacteria that remain sttached to epithelial cells will eventually be swept away as these cells die and are exfoliated. Thus, a proportion of the H. pylori population exists in the nonadherent state. H. pylori must also contend with antibodies and phagocytic cells as the host mounts an immune response [7].
- Transmission
Many researchers think that direct person-to-person contact is the most likely mode of transmission, especially within the family setting. Mother-to-children transmission is known to be more frequent than that of father-to-children transmission; Malaty et al. confirmed that the relative risk of children with H. pylori-positive mothers acquiring infection was 5.3 times that of children whose mothers were H. pylori-negative. In addition to interfamilial transmission, another case-control study found that H. pylori infection may lie outside the family. It is still controversial whether the transmission of the bacterium is gastro-oral, oral-oral, or fecal-oral route. Today, there are many public health measures based on epidemiological data have been extremely successful in preventing the spread of pathogenic agents. Although those measures are successful, the route of transmission of h. pylori should be clarified [8].
- Metabolism
Helicobacter pylori uses glucose as the main source of energy, and the energy is generated through pentose phosphate pathway. The step of metabolic activation by phosphorylation is important in the uptake of saccharides by microorganisms. Covalent attachment of phosphate groups to sugars contribute that it makes the covalent attachment more impermanent to the cell envelope for the intracellular retention of activated saccharides. Phosphorylation of glucose in bacteria is activated by three different kinds of enzymes: hexokinases, glucokinases, and the E-III enzymes of the glucose phosphotransferase system. Pentose phosphate pathway includes both the oxidative and non-oxidative phases; oxidative when producing NADPH and non-oxidative when synthesizing pentose sugars [17].
- Genome Structure
Disease
In human stomach, H. pylori infection can cause diseases such as gastric ulcers and gastritis that can lead to gastric cancer and gastric MALT lymphoma.
- Gastric Ulcer
Gastric ulcer is defined as mucosal erosions equal to or greater than 0.5 cm in stomach. Since H. pylori can survive through the low pH acidity, it can land on the protective mucous lining of the stomach, weakening the protective mucous coating. This allows H. pylori and stomach acid to get into the sensitive mucous lining beneath, causing irritation and formation of ulcer.
- Gastric Cancer
H. pylori infection can also activate the innate and acquired immune responses, as well as effecting changes in the hormonal milieu and acid secretory physiology of the stomach. These changes combine to alter the balance of apoptosis and proliferation and promote the loss of acid-secreting parietal cells. The increase in apoptosis occurs in large part through activation and increased signaling through the Fas/Fas L pathway. Parietal cell loss is compensated by expansion of a less differentiated precursor lineage that is predisposed to give rise to cancer.
- MALT(Mmucosa Associated Lymphoid Tissue) Lymphoma
H. pylori infection can cause inflammation of the lining of the stomach. By normal immunal reaction of the stomach, the inflamation developes MALT-type lymphoid tissues which triggers continuous stimulation of the lymphocytes to replicate and increase in number. However, in a small minority of people, this results in a mistake within the genetic material of a lymphoid cell and continuation of this faulty cell line leads to the development of a lymphoma.
Treatments
Carnivores
Special Environment
The ability of the carnivore stomach to secrete hydrochloric acid is exceptional. Carnivores are able to keep their gastric pH down around 1-2 even with food present. This is necessary to facilitate protein breakdown and to kill the abundant dangerous bacteria often found in decaying flesh foods [9].
Microbes
Bacteria such as salmonella, shigella, Escherichia coli (E.Coli) O157:H7 and other food borne pathogens are skillfully handled by the extended time in the strongly acidic environment of the stomach [10].
Herbivores
Special Environment
Cow is a ruminant which is a foregut fementor with a four-chambered stomach. Four chambers are rumen, reticulum, omasum, and abomasum and among them, abomasum has the most similar function to the stomach of a man [12]. Its pH is normally around 6.0 but because its walls secrete enzymes and hydrochloric acid, the pH is able to be lowered to about 2.5. [13]. To store large quantities of food, there is rumen which is the largest compartment [13]. The environment within the rumen is anaerobic, 39C, with a pH between 6.0 and 7.0. [12]. Due to its high and constant pH, the rumen is the perfect place for the bacteria to live.
Microbes
Rumen bacteria are classified into 4groups; fiber digesters, starch and sugar digesters, lactate using bacteria, and hydrogen-using bacteria [14]. They cooperate together.
Megasphaera elsdenii
It is an anaerobic bacterium which belongs to lactate fermenting species [15]. Megasphaera elsdenii grows using lactic acid and as a result the rumen is cleaned up. This helps to raise the pH of rumen which is good for the growth of the acid-intolerant bacteria [14].
Streptococcus bovis
Streptococcus bovis is one of the most acid-tolerant bacteria among ruminal bacteria. It produces lactic acid when ruminants ingest high-starch diets and pH is low [16]. As mentioned above, Megasphaera elsdenii uses lactic acid which is from Streptococcus bovis to grow and consequently pH is raised. However, because of its quick raising of pH, rumen acidosis can be caused.
Current Research
References
1. O’Rourke, Jani and Bode, Gunter. “Morphology and Ultrastructure.” Helicobacter pylori: Physiology and Genetics, 2001. 6: 53-67.
2. Anderson, L. P. and Wadstrom, Torkel. “Basic Bacteriology and Culture.” Helicobacter pylori: Physiology and Genetics, 2001. 4: 27-38.
3. Solnick, Jay V. and Vandamme, Peter. “Taxonomy of the Helicobacter Genus.” Helicobacter pylori: Physiology and Genetics, 2001. 5: 39-51.
4. Lynch, Nancy A.. “Helicobacter pylori” and Ulcers: a Paradigm Revised.
5. Sachs, George, Scott, David R., Weeks, David L., Rektorscheck, Marina, and Melchers, Klaus. “Regulation of Urease for Acid Habitation.” Helicobacter pylori: Physiology and Genetics, 2001. 25: 277-291.
6. Dubois, Andre. “Spiral Bacteria in the Human Stomach: The Gastric Helicobacters.” Emerging Infectious Diseases, 1995. Vol. 1, No. 3.
7. Testerman, Traci L., McGee, David J., and Mobley, Harry L.. “Adherence and Colonization.” Helicobacter pylori: Physiology and Genetics, 2001. 34: 381-417.
8. Mitchell, Hazel M.. “Epidemiology of Infection.” Helicobacter pylori: Physiology and Genetics, 2001. 2: 7-18.
9. Mills, Milton R. “The Comparative Anatomy of Eating” VegSource Interactive Inc. 26 March 1996.
10. Simpson, JW, and Else, RW. (1991) Digestive Disease in the Dog and Cat. Blackwell Scientific Publications.
11. Martini, Timmons, Tallitsch. Human Anatomy. 5th Edition. San Francisco: Pearson Education Inc. 2006. 662-663.
12. Dehority, B. (2002). Gastrointestinal tracts of herbivores, particularly the ruminant: Anatomy, physiology and microbial digestion of plants. Journal of applied animal research, 21(2), 145-160
13.
14.
15. Marounek, M., Fliegrova, K., and Bartos, S. (1989). Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Applied and Environmental Microbiology, 1570-1573
16. Asanuma, N., Hino, T. (2002). Regulation of fermentation in a ruminal bacterium, Streptococcus bovis, with special reference to rumen acidosis. Animal Science Journal, 73: 313-325(13)
17. Goodwin, C. Steward, and Worsley, BW. (1993) Helicobacter pylori. Biology and Clinical Practice, 115-141
18. Hunt, R., Tytgat, G. (2002) Helicobacter pylori. Basic Mechanisms to Clinical cure 2002, 171-177
