Targeted Gene Therapy Via Lentiviral Vectors
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduction
Gene Therapy Overview
By Drew Albrecht
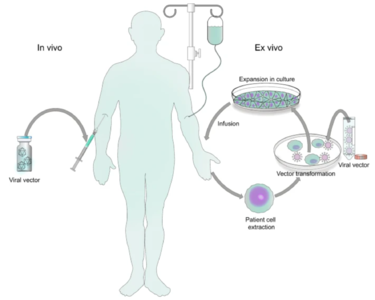
Genetic disorders like cystic fibrosis, phenylketonuria, sickle-cell anemia, and many more, are a result of mutated or absent genes. The goal of gene therapy is to treat or cure such diseases via genetic modification of the cells in an affected individual. Genetic modification can rewire the cells to either produce a therapeutic effect[Kaji] or simply just replace the mutated or missing DNA. Gene therapy can either be ex vivo, where genetic modification of cells occurs outside of the body followed by their transplantation back into the body[Gowing] or in vivo, when genetic information is directly inserted into the body via a vector[Mendell]. Vectors are molecules that aid in the transportation of genetic information all over the body. While both methods prove useful for fighting diseases with genetic modification, in vivo is the more appropriate method for a targeted approach. Performing in vivo gene therapy, there are two different types of vectors: nonviral and viral. Non-viral vectors can be naked DNA, particle based, or chemical based, all transporting DNA throughout the body without the use of a virus. Like the name suggests, viral vectors use the natural tendencies of viruses to infect a host by inserting its DNA into the cytoplasm. Viruses are the perfect carrier to deliver DNA to a target because that is what they do naturally. While non-viral are overall less effective, they are still a promising option due to the low cost, reduced pathogenicity, and ease of production. However, there are a few types of viral vectors that are being tested to drastically change the face of medicine in disease treatment. Currently, the three types of viruses that are being used are adenoviruses (AVs), and adeno-associated viruses (AAVs), and retroviruses, also called lentiviruses.
Viral Vectors
Adenoviruses
Adenoviruses are icosahedral protein capsids that carry a linear, double-stranded DNA genome. These viruses are associated with upper respiratory infection, while occasionally being linked to infection of the bladder and brain. Currently, over a hundred human AV genotypes have been identified and are split into seven subgroups. Upon receptor-mediated entry into the host cell, the DNA is shuttled into the nucleus via nuclear pores. The nucleus is the site of viral gene expression, DNA replication, and progeny production. By taking over the nucleus of the host, the virus gains control over normal cellular functions like immunity responses. By doing so, the cell becomes much more susceptible to infection and cell death[Pied].
AVs have been a hot topic of genetic research for the last five decades due to their advantages as viral vectors. Firstly, AV vectors have a high transduction efficiency in both dividing cells and dormants ones. This means the virus’ ability to infect, kill, and infect the next host is great. A high transduction efficiency is important when dealing with gene therapy because the greater the efficiency of infecting cells, the better the genetic modification will work. Secondly, it has been observed that AV episomal persistence (the amount of time and generations that the AV DNA persists) was long. Thirdly, there is a broad range for different tissues that the AV vector can target. Finally, there is a large scalability of this vector system, production-wise[Bulcha].
AV vectors are derived from the human adenoviruses 2 and 5 (HAd2 and HAd5), the two best understood human AVs. There have been multiple generations of research into AV vectors, with each generation improving on the faults of the previous. Initially, unexpected problems arose from genes in the AV genome, but were later cut out to limit adverse reactions. A recent example of an adenovirus vector in action was with the Johnson & Johnson COVID-19 vaccine. This vaccine was simply a carrier for the gene that codes for the COVID spike protein, so once transcribed and translated, the immune system would produce antibodies. While no genetic modification occurred with this vaccine, it shows how AV vectors are being implemented into the healthcare setting today[Mayoclinic].
Adeno-Associated Viruses
Lentiviruses
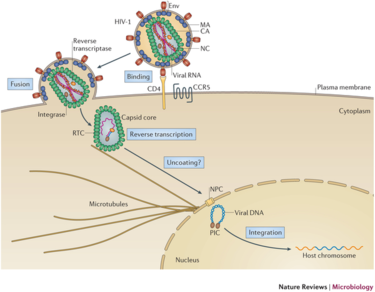
Lentivirus Infection Pathway
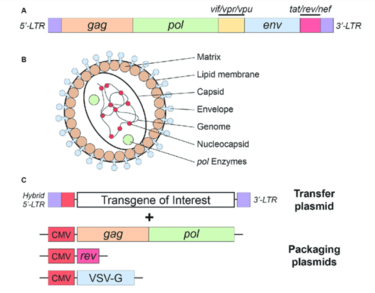
Lentiviral Vectors
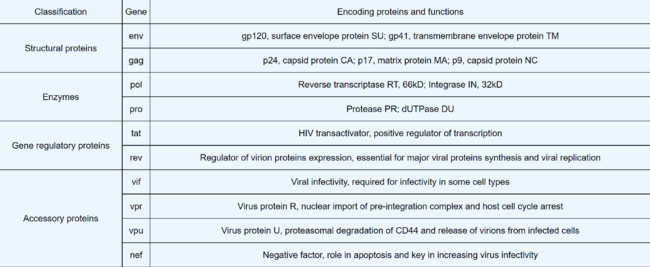
HIV As a Viral Vector
History
Safety
Using HIV To Fight HIV
Current Treatments
Sample citations: [1]
[2]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
To repeat the citation for other statements, the reference needs to have a names: "<ref name=aa>"
The repeated citation works like this, with a forward slash.[1]
