Targeted Gene Therapy Via Lentiviral Vectors
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduction
By Drew Albrecht
Gene therapy is a revolutionary technique that is on the forefront of medical innovation. With the use of targeted gene therapy, disease-specific treatments can be made to cure people of diseases that were once thought to be incurable. There are many methods that are being tested right now to advance gene therapy, but one that is gaining serious traction are lentiviral vectors. Vectors based on viruses are very attractive for gene therapy because viruses are naturally very good at getting into cells. Lentiviruses are even better because they insert their genes into the host’s genomes. With the creation of lentiviral particles with a specific gene of interest, researchers can target affected cells and recover the corrected DNA. This methodology is already being used to treat and cure many diseases today, and the future holds many promises for beating the unbeatable.
Gene Therapy Overview
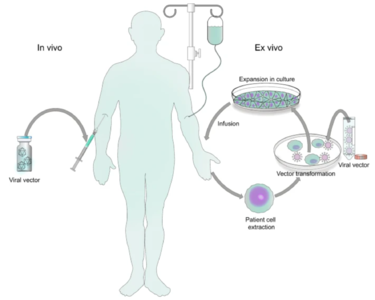
Genetic disorders like cystic fibrosis, phenylketonuria, sickle-cell anemia, and many more, are a result of mutated or absent genes. The goal of gene therapy is to treat or cure such diseases via genetic modification of the cells in an affected individual. Genetic modification can rewire the cells to either produce a therapeutic effect[1] or simply just replace the mutated or missing DNA[2]. Gene therapy can either be ex vivo, where genetic modification of cells occurs outside of the body followed by their transplantation back into the body[3] or in vivo, when genetic information is directly inserted into the body via a vector[4]. Vectors are molecules that aid in the transportation of genetic information all over the body. While both methods prove useful for fighting diseases with genetic modification, in vivo is the more appropriate method for a targeted approach. Performing in vivo gene therapy, there are two different types of vectors: nonviral and viral. Non-viral vectors can be naked DNA, particle based, or chemical based, all transporting DNA throughout the body without the use of a virus. Like the name suggests, viral vectors use the natural tendencies of viruses to infect a host by inserting its DNA into the cytoplasm. Viruses are the perfect carrier to deliver DNA to a target because that is what they do naturally. While non-viral are overall less effective, they are still a promising option due to the low cost, reduced pathogenicity, and ease of production. However, there are a few types of viral vectors that are being tested to drastically change the face of medicine in disease treatment. Currently, the three types of viruses that are being used are adenoviruses (AVs), and adeno-associated viruses (AAVs), and retroviruses, also called lentiviruses.
Viral Vectors
Adenoviruses
Adenoviruses are icosahedral protein capsids that carry a linear, double-stranded DNA genome. These viruses are associated with upper respiratory infection, while occasionally being linked to infection of the bladder and brain. Currently, over a hundred human AV genotypes have been identified and are split into seven subgroups. Upon receptor-mediated entry into the host cell, the DNA is shuttled into the nucleus via nuclear pores. The nucleus is the site of viral gene expression, DNA replication, and progeny production. By taking over the nucleus of the host, the virus gains control over normal cellular functions like immunity responses. By doing so, the cell becomes much more susceptible to infection and cell death[5]. It should be noted that there is no host genome integration.
AVs have been a hot topic of genetic research for the last five decades due to their advantages as viral vectors. Firstly, AV vectors have a high transduction efficiency in both dividing cells and dormants ones. This means the virus’ ability to infect, kill, and infect the next host is great. A high transduction efficiency is important when dealing with gene therapy because the greater the efficiency of infecting cells, the better the genetic modification will work. Secondly, the DNA capacity at which the vector can pack is large (~36 kb). Thirdly, there is a broad range for different tissues that the AV vector can target. Finally, there is a large scalability of this vector system, production-wise[6].
AV vectors are derived from the human adenoviruses 2 and 5 (HAd2 and HAd5), the two best understood human AVs. There have been multiple generations of research into AV vectors, with each generation improving on the faults of the previous. Initially, unexpected problems arose from genes in the AV genome, but were later cut out to limit adverse reactions. A recent example of an adenovirus vector in action was with the Johnson & Johnson COVID-19 vaccine. This vaccine was simply a carrier for the gene that codes for the COVID spike protein, so once transcribed and translated, the immune system would produce antibodies. While no genetic modification occurred with this vaccine, it shows how AV vectors are being implemented into the healthcare setting today[7]. AV based gene therapy clinical trials account for about half of the total worldwide trials, mainly researching new cancer therapies and vaccines.
Adeno-Associated Viruses
Discovered forty years ago, adeno-associated viruses are small protein shells that protect a short, single-stranded DNA genome. They belong to the parvovirus family and depend on co-infection with an adenovirus for replication[8]. Like the AV vectors, recombinant AAV vectors (rAAVs) have been stripped of any harmful genes, leaving a protein shell to transduce the desired gene into the nucleus. rAAVs also do not integrate their DNA into the genome, meaning as multiple rounds of replication undergo, the recombinant episomal DNA will become diluted over time. Persistence of the transgene will be dependent on the turnover rate of the target tissue, making rAAV a great vector choice for timed dosing of any drug. AAV vectors are very similar to their AV counterpart, yet they both have their advantages and disadvantages. AAV has a potential for longer lasting gene expression, but AV has a much greater packaging capacity and faster onset of expression (16-24 hours for AV versus 2-7 days for AAV).
Lentiviruses
Lentivirus Infection Pathway
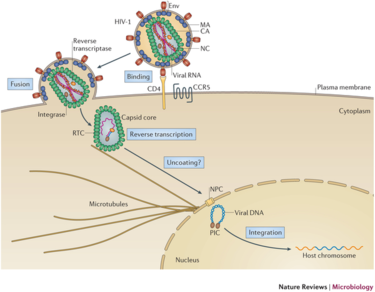
Lentiviral Vectors
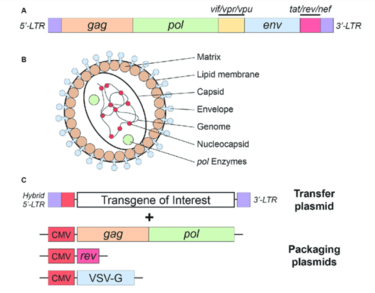
How Do Lentivirus Vectors Work
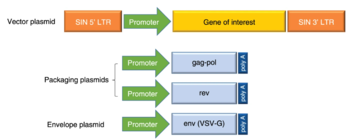
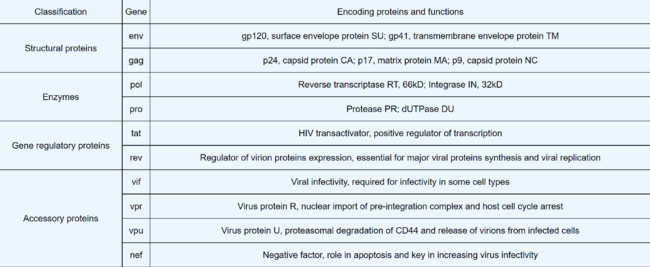
HIV As a Viral Vector
History
Safety
Using HIV To Fight HIV

Current Treatments
Sample citations: [9]
[10]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
To repeat the citation for other statements, the reference needs to have a names: "<ref name=aa>"
The repeated citation works like this, with a forward slash.[9]
- ↑ Kaji EH, Leiden JM. 2001 Gene and stem cell therapies. JAMA 285, 545–550. (doi:10.1001/jama.285.5.545)
- ↑ Lai R. 2022 The Role of Lentiviral Vectors in Treating Lysosomal Storage Diseases. Rare Disease Advisor.
- ↑ Gowing G, Svendsen S, Svendsen CN. 2017 Ex vivo gene therapy for the treatment of neurological disorders. Prog Brain Res 230, 99–132. (doi:10.1016/bs.pbr.2016.11.003)
- ↑ Mendell JR et al. 2021 Current Clinical Applications of In Vivo Gene Therapy with AAVs. Molecular Therapy 29, 464–488. (doi:10.1016/j.ymthe.2020.12.007)
- ↑ Pied N, Wodrich H. 2019 Imaging the adenovirus infection cycle. FEBS Lett 593, 3419–3448. (doi:10.1002/1873-3468.13690)
- ↑ Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. 2021 Viral vector platforms within the gene therapy landscape. Sig Transduct Target Ther 6, 1–24. (doi:10.1038/s41392-021-00487-6)
- ↑ Mayo Clinic. In press. The Johnson & Johnson adenovirus vaccine explained. Mayo Clinic.
- ↑ Naso MF, Tomkowicz B, Perry WL, Strohl WR. 2017 Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 31, 317–334. (doi:10.1007/s40259-017-0234-5)
- ↑ 9.0 9.1 Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
