The History and Transmission of Treponema pallidum; Syphilis: Difference between revisions
| (38 intermediate revisions by the same user not shown) | |||
| Line 11: | Line 11: | ||
==Description of Structure and Significance== | ==Description of Structure and Significance== | ||
<br>By: Marcus Townsend<br> | <br>By: Marcus Townsend<br> | ||
<br>The bacteria <i>Treponema pallidum</i> is a bacteria prominently known for the cause of the chronic sexually transmitted disease syphilis. <i>T. pallidum</i>, has relatives that are in its subspecies. These trepenomes include <i>T. pallidum endemicum</i>, which causes Bejel, <i>T. pallidum pertenue</i>, which causes Yaws, and <i>T. pallidum carateum</i>, which causes Pinta[[#References|[2]]].Phenotypically, these four diseases appear similar as do their serology, but are very distinct based on their unique genotypes. <i>T. pallidum</i> has a circular genome, which is very small in comparison to other prokaryotes. It contains roughly 1000 kilobase pairs in its genome. These distinct diseases are typically found in humans. <i>T. pallidum</i> is classified as a spirochete, and its shape resembles that of a spiraling home telephone cord or staircase (<b>Figure 1</b>). Its structure is very thin, and flexible. <i>T. pallidum</i> is considered a Gram-negative bacterium. Around its spiral shaped body, there is a cytoplasmic membrane which is enclosed by a second outer membrane. In between the cytoplasmic and outer membrane, is a thin layer of peptidoglycan that accounts for stability within the microbe. <i>T. pallidum</i> is versatile because of its ability to be a motile bacterium. The endoflagella located within the periplasmic space between the two membranes, allows the microbe to maneuver its environment in what has been documented as a corkscrew motion. <i>T. pallidum</i> relies on chemotactic responses to be motile. The endoflagella contain 36 gene encoding proteins responsible for the structure and function of the flagella, along with two copies of the flagellar switch protein FliG. <i>T. pallidum</i> ranges in length, from 6-15µm and is about 0.2 µm in width. <i>T. pallidum</i> is a small bacterium that requires a dark field microscopy to be visualized (<b>Figure 1</b>). Other techniques involved for visualizing this microbe include using a modified Steiner silver, the Dieterle stain or the Giemsa stain | <br>The bacteria <i>Treponema pallidum</i> is a bacteria prominently known for the cause of the chronic sexually transmitted disease syphilis. <i>T. pallidum</i>, has relatives that are in its subspecies. These trepenomes include <i>T. pallidum endemicum</i>, which causes Bejel, <i>T. pallidum pertenue</i>, which causes Yaws, and <i>T. pallidum carateum</i>, which causes Pinta[[#References|[2]]].Phenotypically, these four diseases appear similar as do their serology, but are very distinct based on their unique genotypes. <i>T. pallidum</i> has a circular genome, which is very small in comparison to other prokaryotes. It contains roughly 1000 kilobase pairs in its genome. These distinct diseases are typically found in humans. <i>T. pallidum</i> is classified as a spirochete, and its shape resembles that of a spiraling home telephone cord or staircase (<b>Figure 1</b>)[[#References|[2]]]. Its structure is very thin, and flexible[[#References|[2]]][[#References|[3]]]. <i>T. pallidum</i> is considered a Gram-negative bacterium. Around its spiral shaped body, there is a cytoplasmic membrane which is enclosed by a second outer membrane. In between the cytoplasmic and outer membrane, is a thin layer of peptidoglycan that accounts for stability within the microbe. <i>T. pallidum</i> is versatile because of its ability to be a motile bacterium[[#References|[2]]][[#References|[3]]]. The endoflagella located within the periplasmic space between the two membranes, allows the microbe to maneuver its environment in what has been documented as a corkscrew motion. <i>T. pallidum</i> relies on chemotactic responses to be motile[[#References|[2]]]. The endoflagella contain 36 gene encoding proteins responsible for the structure and function of the flagella, along with two copies of the flagellar switch protein FliG[[#References|[8]]]. <i>T. pallidum</i> ranges in length, from 6-15µm and is about 0.2 µm in width[[#References|[2]]]. <i>T. pallidum</i> is a small bacterium that requires a dark field microscopy to be visualized (<b>Figure 1</b>)[[#References|[2]]]. Other techniques involved for visualizing this microbe include using a modified Steiner silver, the Dieterle stain or the Giemsa stain[[#References|[6]]]. | ||
This microbe is an obligate parasite as it needs a host to conduct its metabolic processes and life cycle. To culture <i>T. pallidum</i> under in vitro conditions is difficult to achieve. <i>T. pallidum</i> thrives under low oxygen conditions, ranging from 10-20%, making it microaerophile. Not only is it oxygen sensitive, but it is also temperature dependent, as it prefers temperatures relative to the human body. <i>T.pallidum</i> is a primary pathogen because of its ability to compromise the host’s immune system. As far as metabolic pathways are concerned, it does not contain a pathways to synthesize nucleotides, fatty acids, enzyme cofactors or most amino acids. It lacks genes to metabolize amino acids and fatty acids as a possible energy source. However, it does contain the genes essential for the glycolysis pathway, but lacks genes responsible for processes after glycolysis. <i>T. pallidum</i> lacks genes that would allow products from glycolysis to enter the TCA cycle, which would subsequently lead to the electron transport chain. Lastly, it lacks genes that allows oxidative phosphorylation pathways to occur.[[#References|[ | This microbe is an obligate parasite as it needs a host to conduct its metabolic processes and life cycle. To culture <i>T. pallidum</i> under in vitro conditions is difficult to achieve. <i>T. pallidum</i> thrives under low oxygen conditions, ranging from 10-20%, making it microaerophile. Not only is it oxygen sensitive, but it is also temperature dependent, as it prefers temperatures relative to the human body. <i>T.pallidum</i> is a primary pathogen because of its ability to compromise the host’s immune system. As far as metabolic pathways are concerned, it does not contain a pathways to synthesize nucleotides, fatty acids, enzyme cofactors or most amino acids. It lacks genes to metabolize amino acids and fatty acids as a possible energy source[[#References|[8]]]. However, it does contain the genes essential for the glycolysis pathway, but lacks genes responsible for processes after glycolysis[[#References|[8]]]. <i>T. pallidum</i> lacks genes that would allow products from glycolysis to enter the TCA cycle, which would subsequently lead to the electron transport chain. Lastly, it lacks genes that allows oxidative phosphorylation pathways to occur.[[#References|[8]]] Due to <i>T. pallidum</i> missing integral components for the TCA cycle and the ETC, its one dimensionality, based solely on glucose metabolism via glycolysis, it cannot optimize its energy production. <i>T. pallidum</i> also has a specific set of genes that code for 18 unique ATP-binding cassette transporters with specificity for specific molecules. The limited pathway ability of <i>T. pallidum</i> can be attributed to the small genome it possesses. It is heavily reliant upon the conditions the host provides. These factors make it difficult for scientist to readily culture <i>T. pallidum</i> because of the ideal conditions it requires and the limited pathways it can carry out. | ||
==History and Discovery== | ==History and Discovery== | ||
| Line 19: | Line 19: | ||
<br>Sometime during the 15th century, Europe recognized syphilis as a new disease. There are two main theories about how syphilis came about. The first of the theories is, it is believed that during the time Christopher Columbus and his crew of ships went on their voyage to the New World, they may have contracted the disease and brought it back to Europe. The second theory is, syphilis was already present in Europe but went undiagnosed for years. John Hunter was a prominent physician and venereologist during the 18th century in Scotland. Hunter went on to conduct his own investigation of two rampant diseases gonorrhea, and syphilis. He set out to see if the two diseases were derived from the same etiological agent or if they differed. Hunter intentionally inoculated himself with some pus like fluid from an individual who had a disease. Unknown to Hunter, he inoculated himself with two etiological agents for both gonorrhea and syphilis. His findings would alter the scientific community for years, complicating any knowledge the scientific community already had. Eventually, Philippe Ricord developed his own study on the diseases. In 1838, Philippe Ricord published his astute findings, and identified syphilis and gonorrhea as two distinct diseases. | <br>Sometime during the 15th century, Europe recognized syphilis as a new disease. There are two main theories about how syphilis came about. The first of the theories is, it is believed that during the time Christopher Columbus and his crew of ships went on their voyage to the New World, they may have contracted the disease and brought it back to Europe. The second theory is, syphilis was already present in Europe but went undiagnosed for years. John Hunter was a prominent physician and venereologist during the 18th century in Scotland. Hunter went on to conduct his own investigation of two rampant diseases gonorrhea, and syphilis. He set out to see if the two diseases were derived from the same etiological agent or if they differed. Hunter intentionally inoculated himself with some pus like fluid from an individual who had a disease. Unknown to Hunter, he inoculated himself with two etiological agents for both gonorrhea and syphilis. His findings would alter the scientific community for years, complicating any knowledge the scientific community already had. Eventually, Philippe Ricord developed his own study on the diseases. In 1838, Philippe Ricord published his astute findings, and identified syphilis and gonorrhea as two distinct diseases. | ||
In February of 1905, Franz Eilhard Schulze and his assistant John Siegel believed they had come across the etiological agent responsible for syphilis. However, they speculated the etiological agent they discovered was possibly responsible for other prevalent diseases[[#References|[3]]]. It was first classified as a protozoan with the scientific name <i>Cytorrhyctes luis</i>[[#References|[3]]]. To conclusively dismiss any doubts, the director of the Berlin Imperial Health Service, proposed that Edmond Lesser delve deeper into this theory for a definitive answer. Lesser and colleagues placed Paul Erich Hoffmann an assistant dermatologist, Fritz Schaudinn, and Fred Neufeld an expert in bacteriology in charge of the assignment. Fritz Richard Schauddin attended the Wilhelm University, where he studied zoology. In March of 1905, Schaudinn looked at a papula from a woman’s vulva with secondary syphilis. Under a Zeiss microscope, Schaudinn observed the mannerisms and shape of the <i>T. pallidum</i>. He described the microorganisms as very light, thin, and their motility as back and forth[[#References|[3]]]. Based on these characteristics displayed, the trio named it <i>Spirochaeta pallida</i>[[#References|[3]]]. More research revealed to the trio, that the microbe resided in multiple samples of syphilis ulcers. Neufeld left the group during the time the group thought about publication of their findings. Schauddin and Hoffmann published a provisional work of their studies in April of 1905. | In February of 1905, Franz Eilhard Schulze and his assistant John Siegel believed they had come across the etiological agent responsible for syphilis. However, they speculated the etiological agent they discovered was possibly responsible for other prevalent diseases[[#References|[3]]]. It was first classified as a protozoan with the scientific name <i>Cytorrhyctes luis</i>[[#References|[3]]]. To conclusively dismiss any doubts, the director of the Berlin Imperial Health Service, proposed that Edmond Lesser delve deeper into this theory for a definitive answer. Lesser and colleagues placed Paul Erich Hoffmann an assistant dermatologist, Fritz Schaudinn, and Fred Neufeld an expert in bacteriology in charge of the assignment. Fritz Richard Schauddin attended the Wilhelm University, where he studied zoology. In March of 1905, Schaudinn looked at a papula from a woman’s vulva with secondary syphilis [[#References|[3]]]. Under a Zeiss microscope, Schaudinn observed the mannerisms and shape of the <i>T. pallidum</i>. He described the microorganisms as very light, thin, and their motility as back and forth[[#References|[3]]]. Based on these characteristics displayed, the trio named it <i>Spirochaeta pallida</i>[[#References|[3]]]. More research revealed to the trio, that the microbe resided in multiple samples of syphilis ulcers. Neufeld left the group during the time the group thought about publication of their findings. Schauddin and Hoffmann published a provisional work of their studies in April of 1905 [[#References|[3]]]. | ||
In May of 1905, the duo presented their research to the Berlin Medical society. Their work was repeatedly challenged and many people at the conference fervently believed that <i>Cytorrhyctes luis</i>, was the etiological agent responsible for syphilis. A conclusion was never met at the conference and the investigation was re-opened. Albert Neisser was a well-known venereologist, and very opposed to the findings of Schauddin and Hoffmann. Neisser conducted similar research and realized that the findings of the duo were conclusive and mirrored his results. Future studies showed numerous discoveries of syphilis observed in monkeys inoculated with the bacteria and even in children with congenital syphilis solidifying the Schauddin and Hoffmann’s findings. Schauddin and Hoffman decided to change the scientific name to <i>Treponema pallidum</i>. The medical world eventually, and unanimously concluded that <i>T. pallidum</i> was the etiological agent responsible for syphilis. | In May of 1905, the duo presented their research to the Berlin Medical society [[#References|[3]]]. Their work was repeatedly challenged and many people at the conference fervently believed that <i>Cytorrhyctes luis</i>, was the etiological agent responsible for syphilis [[#References|[3]]]. A conclusion was never met at the conference and the investigation was re-opened. Albert Neisser was a well-known venereologist, and very opposed to the findings of Schauddin and Hoffmann. Neisser conducted similar research and realized that the findings of the duo were conclusive and mirrored his results. Future studies showed numerous discoveries of syphilis observed in monkeys inoculated with the bacteria and even in children with congenital syphilis solidifying the Schauddin and Hoffmann’s findings [[#References|[3]]]. Schauddin and Hoffman decided to change the scientific name to <i>Treponema pallidum</i> [[#References|[3]]]. The medical world eventually, and unanimously concluded that <i>T. pallidum</i> was the etiological agent responsible for syphilis. | ||
In 1906, Schauddin, suffered from gastrointestinal amebian abscesses related to research he was conducting. This circumstance forced him to have an immediate surgery. Complications from the infection and surgery led to his early death on June 22nd, 1906[[#References|[3]]]. | In 1906, Schauddin, suffered from gastrointestinal amebian abscesses related to research he was conducting [[#References|[3]]]. This circumstance forced him to have an immediate surgery. Complications from the infection and surgery led to his early death on June 22nd, 1906[[#References|[3]]]. | ||
[[Image:T.jpg|thumb|300px|right|Figure 2. A picture of a researcher drawing blood from a patient during the Tuskeegee experiment. The picture comes from the CDC website. [http://http://cdc.gov/tuskegee/timeline.htm].]] | [[Image:T.jpg|thumb|300px|right|Figure 2. A picture of a researcher drawing blood from a patient during the Tuskeegee experiment. The picture comes from the CDC website. [http://http://cdc.gov/tuskegee/timeline.htm].]] | ||
In the year 1932, the United States Public Health Service began what was known as the “Tuskegee Study of Untreated Syphilis in the Negro Male” experiment down in rural Tuskegee, Alabama (<b>Figure 2</b>). Their patients came from Macon County, Alabama. The United States Public Health service was interested in studying the history of syphilis. In the study, there were a total of 600 test subjects.[[#References|[10]]]. Researchers conducting the study told their patients they were going to be treated for “bad blood”. The term bad blood refers to number of health related issues including anemia, and fatigue. The study was done without the patient’s informed consent for the treatment. Out of the 600 patients, 399 already had syphilis, and the remaining 201 were syphilis free. The test subjects were told, in return for their participation in the research, they would receive free health-care from the United States government, which also included meals and burial insurance. The research was supposed to last for a period of six months, but continued on for an astonishing forty years. They were promised treatment for bad blood, but unfortunately the researchers did not live up to their promise. | In the year 1932, the United States Public Health Service began what was known as the “Tuskegee Study of Untreated Syphilis in the Negro Male” experiment down in rural Tuskegee, Alabama (<b>Figure 2</b>). Their patients came from Macon County, Alabama. The United States Public Health service was interested in studying the history of syphilis. In the study, there were a total of 600 test subjects.[[#References|[10]]]. Researchers conducting the study told their patients they were going to be treated for “bad blood”. The term bad blood refers to number of health related issues including anemia, and fatigue[[#References|[10]]]. The study was done without the patient’s informed consent for the treatment. Out of the 600 patients, 399 already had syphilis, and the remaining 201 were syphilis free[[#References|[10]]]. The test subjects were told, in return for their participation in the research, they would receive free health-care from the United States government, which also included meals and burial insurance. The research was supposed to last for a period of six months, but continued on for an astonishing forty years[[#References|[10]]]. They were promised treatment for bad blood, but unfortunately the researchers did not live up to their promise. | ||
On July 25th, 1972, a writer for the associated press, Jean Heller released the story that the United States Public Health Service’s research had some questionable ethics involved[[#References|[11]]]. In 1947 penicillin was found to be effective treatment in the disease's early stages, yet during the time of the study action was never taken to provide the patients with appropriate treatments[[#References|[10]]]. The Assistant Secretary for Health and Scientific Affairs Merlin K. Duval appointed an advisory panel to further review the ethics of the study. The advisory panel consisted of nine individuals with backgrounds in law, medicine, and religion. The advisory panel found that the patients did consent to be screened and treated. On the other hand, it was concluded the researchers never told the patients what the real intent of the study was or any of the possible consequences associated with the study[[#References|[11]]]. The patients were never notified by the researchers that there was a chance for mortality. The information the patients received was concluded to be misleading and inconclusive for the patients to give informed consent. Even with the latest treatment of penicillin, there was no evidence that researchers either suggested the option of penicillin to patients or the option to discontinue the study. The study was immediately stopped in October of 1972, cited as being unethical and in-humane. In the following year a class-action lawsuit was filed for the patients still alive and the families of the deceased. Then in 1974, a settlement of 10 million dollars was reached for those | On July 25th, 1972, a writer for the associated press, Jean Heller released the story that the United States Public Health Service’s research had some questionable ethics involved[[#References|[11]]]. In 1947 penicillin was found to be effective treatment in the disease's early stages, yet during the time of the study action was never taken to provide the patients with appropriate treatments[[#References|[10]]]. The Assistant Secretary for Health and Scientific Affairs Merlin K. Duval appointed an advisory panel to further review the ethics of the study. The advisory panel consisted of nine individuals with backgrounds in law, medicine, and religion. The advisory panel found that the patients did consent to be screened and treated. On the other hand, it was concluded the researchers never told the patients what the real intent of the study was or any of the possible consequences associated with the study[[#References|[11]]]. The patients were never notified by the researchers that there was a chance for mortality. The information the patients received was concluded to be misleading and inconclusive for the patients to give informed consent. Even with the latest treatment of penicillin, there was no evidence that researchers either suggested the option of penicillin to patients or the option to discontinue the study. The study was immediately stopped in October of 1972, cited as being unethical and in-humane. In the following year a class-action lawsuit was filed for the patients still alive and the families of the deceased. Then in 1974, a settlement of 10 million dollars was reached for those[[#References|[10]]]. The United States government once again promised lifetime health benefits to those who were still living with the disease as well as burial service[[#References|[11]]]. In 1975 it was expanded to cover loved ones of the effected in the study. The last study patient under the Tuskegee experiments died in 2004[[#References|[11]]]. | ||
==Transmission and Symptoms== | ==Transmission and Symptoms== | ||
<br>There are multiple ways in which syphilis is transferred. The primary way is through the action of intercourse, in which there is bacterial transfer to another viable host. The second most common is seen in utero from mother to baby (congenital syphilis) where the bacteria is able to go through the placenta and make contact with the fetus. The last way is through accidental inoculation, in which there is some contact but not necessarily in a sexual manner. <i>T. pallidum</i> looks for a way to breach into cells for infection. <i>T. pallidum</i>’s course of action is able to penetrate dermal tissue that has abrasions, or through an intact mucous membrane. <i>T. pallidum</i> works by attaching to the host cell of interest by using adhesion starting the initial course of infection. | <br>There are multiple ways in which syphilis is transferred. The primary way is through the action of intercourse, in which there is bacterial transfer to another viable host[[#References|[4]]]. The second most common is seen in utero from mother to baby (congenital syphilis) where the bacteria is able to go through the placenta and make contact with the fetus[[#References|[2]]][[#References|[4]]]. The last way is through accidental inoculation, in which there is some contact but not necessarily in a sexual manner. <i>T. pallidum</i> looks for a way to breach into cells for infection. <i>T. pallidum</i>’s course of action is able to penetrate dermal tissue that has abrasions, or through an intact mucous membrane. <i>T. pallidum</i> works by attaching to the host cell of interest by using adhesion starting the initial course of infection. | ||
[[Image:Chancre.jpg|thumb|300px|right|Figure 3. A cartoon of a chancre on the penis caused by <i>Treponema pallidum</i> via intercourse. This is one of the first signs associated the primary stage of syphilis. Chancres are not exclusive to the genitals they can appear on all parts of the body through which <i>Treponema pallidum</i> entered. The cartoon is courtesy of the Mayo Foundation for Medical Education and Research.[http://http://www.drugs.com/mcd/syphilis].]] | [[Image:Chancre.jpg|thumb|300px|right|Figure 3. A cartoon of a chancre on the penis caused by <i>Treponema pallidum</i> via intercourse. This is one of the first signs associated the primary stage of syphilis. Chancres are not exclusive to the genitals they can appear on all parts of the body through which <i>Treponema pallidum</i> entered. The cartoon is courtesy of the Mayo Foundation for Medical Education and Research.[http://http://www.drugs.com/mcd/syphilis].]] | ||
| Line 39: | Line 39: | ||
[[Image:Secondary Syphilis.jpg|thumb|300px|right|Figure 4. A photograph of an individual with symptoms of secondary syphilis. Spots cover the palms of the hand and feet also known as palmar lesions. This photo was taken by the CDC. [http://http://cdc.gov/std/syphilis/images/rash-palmar.htm].]] | [[Image:Secondary Syphilis.jpg|thumb|300px|right|Figure 4. A photograph of an individual with symptoms of secondary syphilis. Spots cover the palms of the hand and feet also known as palmar lesions. This photo was taken by the CDC. [http://http://cdc.gov/std/syphilis/images/rash-palmar.htm].]] | ||
Syphilis has a total of four stages, which include primary syphilis, secondary syphilis, latent syphilis, and late syphilis. During the primary stage of syphilis, <i>T. pallidum</i> has entered the body. <i>T. pallidum</i> works by attaching to the host cell of interest by using adhesion. Eventually sores, or chancres on the genitals will form at the site in which the bacterium has entered the body. The sores and chancres are painless, firm, and usually round. The first symptoms can take between 10-90 days to show. The sores usually last between three to six weeks and then the body will begin to heal. This will then cause a painless sore at the site in which T. pallidum entered the host. If untreated it will progress to the secondary phase. During the secondary phase, rough rashes and sores will start to develop on your body and even in the mouth. There is the possibility of developing spots on your hands and feet, along with an immune responses such as swelling of the lymph nodes, fever, a sore throat, hair loss, noticeable weight loss, body aches, and unusual fatigue. If syphilis is still not treated it will progress to the latent phase. In the latent phase of syphilis, the symptoms experienced in in the primary and secondary stage will eventually go away. Syphilis is a tricky disease. There could be a large period of time for years in which you notice none of the symptoms, as they do not appear in the latent phase. In the late phase which is typically uncommon, syphilis can lead to a reduction in coordination of your body especially in muscular movements. In some cases people who have syphilis paired with HIV have experienced nervous system impairment such as paralysis, loss of eye-sight, or even the formation of mental diseases like dementia. Syphilis has been known to shut down vital organs if the individual reaches the late stage of syphilis without proper treatment. In congenital syphilis, it has been documented that up to two-thirds of new-born babies display no signs of the disease. However, syphilis is | Syphilis has a total of four stages, which include primary syphilis, secondary syphilis, latent syphilis, and late syphilis. During the primary stage of syphilis, <i>T. pallidum</i> has entered the body. <i>T. pallidum</i> works by attaching to the host cell of interest by using adhesion. Eventually sores, or chancres on the genitals will form at the site in which the bacterium has entered the body. The sores and chancres are painless, firm, and usually round (<b>Figure 3</b>)[[#References|[2]]]. The first symptoms can take between 10-90 days to show. The sores usually last between three to six weeks and then the body will begin to heal[[#References|[2]]]. This will then cause a painless sore at the site in which <i>T. pallidum</i> entered the host. If untreated it will progress to the secondary phase. During the secondary phase, rough rashes and sores will start to develop on your body and even in the mouth. There is the possibility of developing spots on your hands and feet (<b>Figure 4</b>), along with an immune responses such as swelling of the lymph nodes, fever, a sore throat, hair loss, noticeable weight loss, body aches, and unusual fatigue[[#References|[2]]]. If syphilis is still not treated it will progress to the latent phase. In the latent phase of syphilis, the symptoms experienced in in the primary and secondary stage will eventually go away. Syphilis is a tricky disease. There could be a large period of time for years in which you notice none of the symptoms, as they do not appear in the latent phase. In the late phase which is typically uncommon, syphilis can lead to a reduction in coordination of your body especially in muscular movements. In some cases people who have syphilis paired with HIV have experienced nervous system impairment such as paralysis, loss of eye-sight, or even the formation of mental diseases like dementia[[#References|[2]]]. Syphilis has been known to shut down vital organs if the individual reaches the late stage of syphilis without proper treatment. In congenital syphilis, it has been documented that up to two-thirds of new-born babies display no signs of the disease. Sometimes still births, and premature births may occur[[#References|[2]]]. However, for the children that survive, syphilis is known to physically show within the first two years of a child's life[[#References|[2]]][[#References|[14]]]. These symptoms include an abnormally large liver, rashes, and inflammation of lungs. Around 40% of children develop the late stages of syphilis, which can lead to saddle nose formation and other[[#References|[14]]]. The major issues when it comes to diagnosing syphilis, is the traditional symptoms people develop are often similar to other diseases, which leads to improper treatment for the patient. In today’s world we have tests that readily allow us to detect syphilis. These include antibody test by drawing blood, a non-treponomal antibody test, a rapid plasma regain, the venereal disease research laboratory test, and physical examination. | ||
==Incidence== | ==Incidence== | ||
<br>Most syphilis cases are usually seen in individuals in their late teens to early thirties. The incidence rate for syphilis is about 1 in every | <br>Most syphilis cases are usually seen in individuals in their late teens to early thirties. The incidence rate for syphilis is about 1 in every 3,885 people in the United States[[#References|[12]]]. It has been estimated that nearly 70,000 intercourse related transmitted infections happen per year in the United States[[#References|[12]]]. Worldwide incidence is around 12 million people annually. The cause is due to third world countries lacking sufficient resources and most importantly educating people. An estimation of deaths per year dating back to 1999 says on average, in the United States, 32 people die per year. Based on the World Health Organization statistics from 2002, mortality globally was around 155,000 people[[#References|[12]]]. | ||
==Research for a Cure and Conclusion== | ==Research for a Cure and Conclusion== | ||
<br>Around the 1900’s, Paul Erlich and colleagues developed a drug that utilized via chemotherapy. Salvarsan a potentially harmful drug was discovered to have some antimicrobial properties effective enough to kill the parasite the drugs name was Salvarsan. Prior to this era other harmful drugs were used such as mercury compounds along with numerous compounds, but they were never proven to be effective. Just like mercury, Slavarsan had a downside. Salvarsan is an arsenical drug which could lead to a number of health related issues including death. The main issue with this drug was that it wasn’t target specific for the bacterium, it could easily attack the host which is what they wanted to avoid. Salvarsan was difficult to administer, and for it to be effective it required multiple treatments over a prolonged period of time. John Mahoney, AD Harris, and Richard Arnold began research in the 1940’s on possible syphilis treatments, after reading a paper produced by Wallace Herrell, and colleagues that worked at the Mayo Clinic. Wallace Herrell’s paper described the effectiveness of penicillin against a strain of sulfonamide-resistant gonorrhea. Mahoney and his group of colleagues began a study at the US Marine Hospital in New York, where they actively treat four patients with primary stage of syphilis. The penicillin was administered by an intramuscular injection four times per hour, over an eight day period. Today, this same technique is used but only requires one injection of Benzathine penicillin G for people going through the primary, secondary or latent phase of the disease. Penicillin has been used to cure patients with syphilis for over 70 years, surprisingly, syphilis is still very sensitive to this form of treatment as there have not been any reported signs of evolving <i>T. pallidum</i> to form any antibiotic resistant strain. Azithromycin is another drug of choice which can be used. This version is taken orally as opposed to receiving shots of penicillin. The rabbit is the model organism of choice when it comes to research for a vaccine as they have a form of syphilis that is similar to the human form. Dr. James Miller in 1973, created an immunization protocol for a set of rabbits which received a total of 60 γ-irradiated shots of <i>T. pallidum</i> over a 37-week period, which was followed by intradermal Nichols strain <i>T. pallidum</i>. He observed that the rabbits that received these injections showed no signs of typical symptoms associated with syphilis at the injection sites. He also noticed there was no infection in naïve animals receiving lymph nodes from rabbits that had been immunized. The immunization process of the rabbits proved to be successful for up to a year after the final immunization received. The protective immunity against re-infection of the host showed that re-infection could occur homologs of <i>T. pallidum</i>, but the process of re-infection occurs at a slower rate in the animal model of the rabbit. This rather slower re-infection can be due to the rabbit’s tuned immune system that was treated heavily with the strain of <i>T. pallidum</i>. The immune system has some recognition of the bacterium slowing down the rate of infection. This outstanding research was ground-breaking as Dr. John Miller was able to find a temporary vaccination for the model species, but on the other hand, this method was not feasible for humans. Finding a cure has not been as simple as many researchers have tried and had moderate success. Being able to culture <i>T. pallidum</i> under lab conditions has proven to be difficult and cumbersome. <i>T. pallidum</i> requires such unique conditions from temperature to pH and even levels of oxygen it can be exposed to. This difficulty exhibited in the lab makes it difficult to formulate a reliable vaccine. Like most viruses we are immune to, they’ve been attenuated for use. As for <i>T. pallidum</i> if it does not have a mammalian host to infect, it is unable to carry out its life cycle making it difficult to observe in an artificial environment. Although <i>T. pallidum</i> is a nuissance it poses no true threat if caught early. There have also been no documented cases of mutated forms resistant to penicillin. However, it has been documented that the country of Cuba has eliminated the transmission of syphilis between mother and child. | <br>Around the 1900’s, Paul Erlich and colleagues developed a drug that utilized via chemotherapy. Salvarsan a potentially harmful drug was discovered to have some antimicrobial properties effective enough to kill the parasite the drugs name was Salvarsan. Prior to this era other harmful drugs were used such as mercury compounds along with numerous compounds, but they were never proven to be effective[[#References|[9]]]. Just like mercury, Slavarsan had a downside. Salvarsan is an arsenical drug which could lead to a number of health related issues including death. The main issue with this drug was that it wasn’t target specific for the bacterium, it could easily attack the host which is what they wanted to avoid. Salvarsan was difficult to administer, and for it to be effective it required multiple treatments over a prolonged period of time[[#References|[9]]]. John Mahoney, AD Harris, and Richard Arnold began research in the 1940’s on possible syphilis treatments, after reading a paper produced by Wallace Herrell, and colleagues that worked at the Mayo Clinic. Wallace Herrell’s paper described the effectiveness of penicillin against a strain of sulfonamide-resistant gonorrhea. Mahoney and his group of colleagues began a study at the US Marine Hospital in New York, where they actively treat four patients with primary stage of syphilis. The penicillin was administered by an intramuscular injection four times per hour, over an eight day period. Today, this same technique is used but only requires one injection of Benzathine penicillin G for people going through the primary, secondary or latent phase of the disease[[#References|[15]]]. Penicillin has been used to cure patients with syphilis for over 70 years, surprisingly, syphilis is still very sensitive to this form of treatment as there have not been any reported signs of evolving <i>T. pallidum</i> to form any antibiotic resistant strain. Azithromycin is another drug of choice which can be used[[#References|[15]]]. This version is taken orally as opposed to receiving shots of penicillin. The rabbit is the model organism of choice when it comes to research for a vaccine as they have a form of syphilis that is similar to the human form. Dr. James Miller in 1973, created an immunization protocol for a set of rabbits which received a total of 60 γ-irradiated shots of <i>T. pallidum</i> over a 37-week period, which was followed by intradermal Nichols strain <i>T. pallidum</i>. He observed that the rabbits that received these injections showed no signs of typical symptoms associated with syphilis at the injection sites. He also noticed there was no infection in naïve animals receiving lymph nodes from rabbits that had been immunized. The immunization process of the rabbits proved to be successful for up to a year after the final immunization received. The protective immunity against re-infection of the host showed that re-infection could occur homologs of <i>T. pallidum</i>, but the process of re-infection occurs at a slower rate in the animal model of the rabbit. This rather slower re-infection can be due to the rabbit’s tuned immune system that was treated heavily with the strain of <i>T. pallidum</i>. The immune system has some recognition of the bacterium slowing down the rate of infection. This outstanding research was ground-breaking as Dr. John Miller was able to find a temporary vaccination for the model species, but on the other hand, this method was not feasible for humans. | ||
[[Image:K.jpg|thumb|300px|right|Figure 5. Biopsy examination correlation with the duration of secondary syphilis lesions with skin erruptions. Pictures A-D indicate the duration of lesions in days. The study was done by Stary et al. [http://http://sciencedirect.com/science/article/pii/S0002944010602945].]] | |||
In a paper written by Stary et al., the group of researchers investigated the human immune system defense mechanism against secondary syphilis lesions.There were a total of 25 patients, 13 of which were HIV positive. Individuals with syphilis and HIV have a lower CD4+ T-cell count versus those with just syphilis. A biopsy was performed on each patient; these samples were classified based on the duration of the secondary syphilis symptoms (0-10 days, 11-20 days, 21-30 days, >30 days)[[#References|[16]]]. Individuals with HIV had also had a higher count in the number of <i>T. pallidum</i> present. The innate immune system would respond sending macrophages, and neutrophils to sites of infection. They observed that the HIV negative group had CD4 and CD8 T-cells present while the HIV positives had only CD8 T-cells present(<b>Figure 5</b>). This is most likely due to having concurrent diseases. It was concluded that both CD4 and CD8 T-cells are important for combating syphilis. They then compared these biopsies graphically showing the differences in acanthosis, dykeratosis, plasma cells and nodular infiltrate of the differing time periods. There appeared to be a rather clear trend for acanthosis plasma cells nodular infiltrates; the longer the individual had secondary syphilis there a clear increase in all those three observable variables (<b>Figure 5</b>)[[#References|[16]]]. The individuals were then clustered into 4 groups depending on how long they've displayed secondary symptoms. | |||
[[Image:j.jpg|thumb|300px|right|Figure 6. Quantitative RT-PCR of biopsies and the regualtion of cytokines. The study was done by Stary et al. [http://http://sciencedirect.com/science/article/pii/S0002944010602945].]] | |||
Stary et al., conducted more research to find the prevalence of cytokines in the biopsies using quantitative RT-PCR.IFN-y mRNA was in syphilitic skin was up-regulated in comparison to normal healthy skin (<b>Figure 6</b>)[[#References|[16]]]. The quantitative RT-PCR revealed, HIV positive individuals with lesions, had a larger up-regulation compared to HIV negative individuals. This difference however, was not observed between lesions of different time stages. IFN-y, IL-23p19, IL-17, and IL-22 were all up regulated in HIV positive patients[[#References|[16]]]. They concluded the T-cell producing cytokines for Th1 and Th17 in the secondary syphilis stage are vastly important for fighting the infection of <i>T. pallidum</i> leading to the amplified response[[#References|[16]]]. | |||
Finding a cure has not been as simple as many researchers have tried and had moderate success. Being able to culture <i>T. pallidum</i> under lab conditions has proven to be difficult and cumbersome[[#References|[2]]]. <i>T. pallidum</i> requires such unique conditions from temperature to pH and even levels of oxygen it can be exposed to. This difficulty exhibited in the lab makes it difficult to formulate a reliable vaccine[[#References|[2]]]. Like most viruses we are immune to, they’ve been attenuated for use. As for <i>T. pallidum</i> if it does not have a mammalian host to infect, it is unable to carry out its life cycle making it difficult to observe in an artificial environment. Although <i>T. pallidum</i> is a nuissance it poses no true threat if caught early. There have also been no documented cases of mutated forms resistant to penicillin. However, it has been documented that the country of Cuba has eliminated the transmission of syphilis between mother and child [[#References|[13]]]. | |||
==References== | ==References== | ||
| Line 52: | Line 63: | ||
<br><br>Authored for BIOL 238 Microbiology, taught by [mailto:slonczewski@kenyon.edu Joan Slonczewski], 2016, [http://www.kenyon.edu/index.xml Kenyon College]. | <br><br>Authored for BIOL 238 Microbiology, taught by [mailto:slonczewski@kenyon.edu Joan Slonczewski], 2016, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
1.Tampa M., | 1.Tampa M., et al., (2014). “Brief History of Syphilis“ Journal of Medicine and Life 7(1):4-10 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3956094/ | ||
2.LaFond R.E., Lukehart S. (2006). “Biological Basis for Syphilis” Clinical Microbiology Review 19(1):29-49 http://cmr.asm.org/content/19/1/29.full?view=long&pmid=16418521#sec-1 | 2.LaFond R.E., Lukehart S., (2006). “Biological Basis for Syphilis” Clinical Microbiology Review 19(1):29-49 http://cmr.asm.org/content/19/1/29.full?view=long&pmid=16418521#sec-1 | ||
3.Souza de E.M.,(2005). “A hundred years ago, the discovery of Treponema pallidum” 80(5):547-8 http://www.scielo.br/pdf/abd/v80n5/en_v80n5a17.pdf | 3.Souza de E.M.,(2005). “A hundred years ago, the discovery of Treponema pallidum” 80(5):547-8 http://www.scielo.br/pdf/abd/v80n5/en_v80n5a17.pdf | ||
4.Cameron C.E., Lukehart S. (2014). “Current Status of Syphilis Vaccine Development: Need, Challenges, Prospects” Vaccine 32(14): 1602-1609 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3951677/ | 4.Cameron C.E., Lukehart S., (2014). “Current Status of Syphilis Vaccine Development: Need, Challenges, Prospects” Vaccine 32(14): 1602-1609 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3951677/ | ||
5.Schwartz W.F., (1961) Public Health Image Library http://phil.cdc.gov/phil/details.asp?pid=10179#modalIdString_CDCImage_0 | 5.Schwartz W.F., (1961) Public Health Image Library http://phil.cdc.gov/phil/details.asp?pid=10179#modalIdString_CDCImage_0 | ||
| Line 71: | Line 82: | ||
https://www.researchgate.net/publication/16188760_The_Theoretical_Basis_of_Paul_Ehrlich's_Chemotherapy | https://www.researchgate.net/publication/16188760_The_Theoretical_Basis_of_Paul_Ehrlich's_Chemotherapy | ||
10.http://www.cdc.gov/ | 10."The Tuskeegee Timeline" 19 February 2016. | ||
http://www.cdc.gov/tuskegee/timeline.htm | |||
11.McCallum J.M., et al., (2006). “Awareness and Knowledge of the U.S. Public Health Service Syphilis Study at Tuskegee: Implications for Biomedical Research” Journal of Health Care Poor Underserved 17(4): 716-733 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1828138/ | 11.McCallum J.M., et al., (2006). “Awareness and Knowledge of the U.S. Public Health Service Syphilis Study at Tuskegee: Implications for Biomedical Research” Journal of Health Care Poor Underserved 17(4): 716-733 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1828138/ | ||
12.http://www.rightdiagnosis.com/s/syphilis/deaths.htm | 12."Deaths from Syphilis" 13 August 2015. | ||
http://www.rightdiagnosis.com/s/syphilis/deaths.htm | |||
13."WHO validates elimination of mother-to-child transmission of HIV and syphilis in Cuba" 30 June 2015. http://www.who.int/mediacentre/news/releases/2015/mtct-hiv-cuba/en/ | 13."WHO validates elimination of mother-to-child transmission of HIV and syphilis in Cuba" 30 June 2015. http://www.who.int/mediacentre/news/releases/2015/mtct-hiv-cuba/en/ | ||
14.Woods R.C.,(2009). "Congenital Syphilis-Persisting Pestilence" Pediatric Infectious Disease Journal 28(6):536-7. http://journals.lww.com/pidj/Citation/2009/06000/Congenital_Syphilis_Persisting_Pestilence.16.aspx | |||
15. "Syphilis Treatment and Care" 3 December 2015. http://www.cdc.gov/std/syphilis/treatment.htm | |||
16. Stary R.C., et al., (2010). "Host Defense Mechanisms in Secondary Syphilitic Lesions : A Role for IFN-γ-/IL-17–Producing CD8+ T Cells?" The American Journal of Pathology 177(5):2421–2432 | |||
http://www.sciencedirect.com/science/article/pii/S0002944010602945 | |||
Latest revision as of 18:19, 13 May 2016
Classification of Treponema pallidum
• Kingdom: Bacteria
• Phylum: Spirochaetae
• Class: Spirochaetes
• Order: Spirochaetales
• Family: Spirochaetacecae
• Genus: Treponema [2]
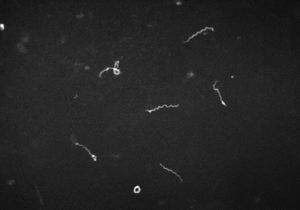
Description of Structure and Significance
By: Marcus Townsend
The bacteria Treponema pallidum is a bacteria prominently known for the cause of the chronic sexually transmitted disease syphilis. T. pallidum, has relatives that are in its subspecies. These trepenomes include T. pallidum endemicum, which causes Bejel, T. pallidum pertenue, which causes Yaws, and T. pallidum carateum, which causes Pinta[2].Phenotypically, these four diseases appear similar as do their serology, but are very distinct based on their unique genotypes. T. pallidum has a circular genome, which is very small in comparison to other prokaryotes. It contains roughly 1000 kilobase pairs in its genome. These distinct diseases are typically found in humans. T. pallidum is classified as a spirochete, and its shape resembles that of a spiraling home telephone cord or staircase (Figure 1)[2]. Its structure is very thin, and flexible[2][3]. T. pallidum is considered a Gram-negative bacterium. Around its spiral shaped body, there is a cytoplasmic membrane which is enclosed by a second outer membrane. In between the cytoplasmic and outer membrane, is a thin layer of peptidoglycan that accounts for stability within the microbe. T. pallidum is versatile because of its ability to be a motile bacterium[2][3]. The endoflagella located within the periplasmic space between the two membranes, allows the microbe to maneuver its environment in what has been documented as a corkscrew motion. T. pallidum relies on chemotactic responses to be motile[2]. The endoflagella contain 36 gene encoding proteins responsible for the structure and function of the flagella, along with two copies of the flagellar switch protein FliG[8]. T. pallidum ranges in length, from 6-15µm and is about 0.2 µm in width[2]. T. pallidum is a small bacterium that requires a dark field microscopy to be visualized (Figure 1)[2]. Other techniques involved for visualizing this microbe include using a modified Steiner silver, the Dieterle stain or the Giemsa stain[6].
This microbe is an obligate parasite as it needs a host to conduct its metabolic processes and life cycle. To culture T. pallidum under in vitro conditions is difficult to achieve. T. pallidum thrives under low oxygen conditions, ranging from 10-20%, making it microaerophile. Not only is it oxygen sensitive, but it is also temperature dependent, as it prefers temperatures relative to the human body. T.pallidum is a primary pathogen because of its ability to compromise the host’s immune system. As far as metabolic pathways are concerned, it does not contain a pathways to synthesize nucleotides, fatty acids, enzyme cofactors or most amino acids. It lacks genes to metabolize amino acids and fatty acids as a possible energy source[8]. However, it does contain the genes essential for the glycolysis pathway, but lacks genes responsible for processes after glycolysis[8]. T. pallidum lacks genes that would allow products from glycolysis to enter the TCA cycle, which would subsequently lead to the electron transport chain. Lastly, it lacks genes that allows oxidative phosphorylation pathways to occur.[8] Due to T. pallidum missing integral components for the TCA cycle and the ETC, its one dimensionality, based solely on glucose metabolism via glycolysis, it cannot optimize its energy production. T. pallidum also has a specific set of genes that code for 18 unique ATP-binding cassette transporters with specificity for specific molecules. The limited pathway ability of T. pallidum can be attributed to the small genome it possesses. It is heavily reliant upon the conditions the host provides. These factors make it difficult for scientist to readily culture T. pallidum because of the ideal conditions it requires and the limited pathways it can carry out.
History and Discovery
Sometime during the 15th century, Europe recognized syphilis as a new disease. There are two main theories about how syphilis came about. The first of the theories is, it is believed that during the time Christopher Columbus and his crew of ships went on their voyage to the New World, they may have contracted the disease and brought it back to Europe. The second theory is, syphilis was already present in Europe but went undiagnosed for years. John Hunter was a prominent physician and venereologist during the 18th century in Scotland. Hunter went on to conduct his own investigation of two rampant diseases gonorrhea, and syphilis. He set out to see if the two diseases were derived from the same etiological agent or if they differed. Hunter intentionally inoculated himself with some pus like fluid from an individual who had a disease. Unknown to Hunter, he inoculated himself with two etiological agents for both gonorrhea and syphilis. His findings would alter the scientific community for years, complicating any knowledge the scientific community already had. Eventually, Philippe Ricord developed his own study on the diseases. In 1838, Philippe Ricord published his astute findings, and identified syphilis and gonorrhea as two distinct diseases.
In February of 1905, Franz Eilhard Schulze and his assistant John Siegel believed they had come across the etiological agent responsible for syphilis. However, they speculated the etiological agent they discovered was possibly responsible for other prevalent diseases[3]. It was first classified as a protozoan with the scientific name Cytorrhyctes luis[3]. To conclusively dismiss any doubts, the director of the Berlin Imperial Health Service, proposed that Edmond Lesser delve deeper into this theory for a definitive answer. Lesser and colleagues placed Paul Erich Hoffmann an assistant dermatologist, Fritz Schaudinn, and Fred Neufeld an expert in bacteriology in charge of the assignment. Fritz Richard Schauddin attended the Wilhelm University, where he studied zoology. In March of 1905, Schaudinn looked at a papula from a woman’s vulva with secondary syphilis [3]. Under a Zeiss microscope, Schaudinn observed the mannerisms and shape of the T. pallidum. He described the microorganisms as very light, thin, and their motility as back and forth[3]. Based on these characteristics displayed, the trio named it Spirochaeta pallida[3]. More research revealed to the trio, that the microbe resided in multiple samples of syphilis ulcers. Neufeld left the group during the time the group thought about publication of their findings. Schauddin and Hoffmann published a provisional work of their studies in April of 1905 [3].
In May of 1905, the duo presented their research to the Berlin Medical society [3]. Their work was repeatedly challenged and many people at the conference fervently believed that Cytorrhyctes luis, was the etiological agent responsible for syphilis [3]. A conclusion was never met at the conference and the investigation was re-opened. Albert Neisser was a well-known venereologist, and very opposed to the findings of Schauddin and Hoffmann. Neisser conducted similar research and realized that the findings of the duo were conclusive and mirrored his results. Future studies showed numerous discoveries of syphilis observed in monkeys inoculated with the bacteria and even in children with congenital syphilis solidifying the Schauddin and Hoffmann’s findings [3]. Schauddin and Hoffman decided to change the scientific name to Treponema pallidum [3]. The medical world eventually, and unanimously concluded that T. pallidum was the etiological agent responsible for syphilis.
In 1906, Schauddin, suffered from gastrointestinal amebian abscesses related to research he was conducting [3]. This circumstance forced him to have an immediate surgery. Complications from the infection and surgery led to his early death on June 22nd, 1906[3].

In the year 1932, the United States Public Health Service began what was known as the “Tuskegee Study of Untreated Syphilis in the Negro Male” experiment down in rural Tuskegee, Alabama (Figure 2). Their patients came from Macon County, Alabama. The United States Public Health service was interested in studying the history of syphilis. In the study, there were a total of 600 test subjects.[10]. Researchers conducting the study told their patients they were going to be treated for “bad blood”. The term bad blood refers to number of health related issues including anemia, and fatigue[10]. The study was done without the patient’s informed consent for the treatment. Out of the 600 patients, 399 already had syphilis, and the remaining 201 were syphilis free[10]. The test subjects were told, in return for their participation in the research, they would receive free health-care from the United States government, which also included meals and burial insurance. The research was supposed to last for a period of six months, but continued on for an astonishing forty years[10]. They were promised treatment for bad blood, but unfortunately the researchers did not live up to their promise.
On July 25th, 1972, a writer for the associated press, Jean Heller released the story that the United States Public Health Service’s research had some questionable ethics involved[11]. In 1947 penicillin was found to be effective treatment in the disease's early stages, yet during the time of the study action was never taken to provide the patients with appropriate treatments[10]. The Assistant Secretary for Health and Scientific Affairs Merlin K. Duval appointed an advisory panel to further review the ethics of the study. The advisory panel consisted of nine individuals with backgrounds in law, medicine, and religion. The advisory panel found that the patients did consent to be screened and treated. On the other hand, it was concluded the researchers never told the patients what the real intent of the study was or any of the possible consequences associated with the study[11]. The patients were never notified by the researchers that there was a chance for mortality. The information the patients received was concluded to be misleading and inconclusive for the patients to give informed consent. Even with the latest treatment of penicillin, there was no evidence that researchers either suggested the option of penicillin to patients or the option to discontinue the study. The study was immediately stopped in October of 1972, cited as being unethical and in-humane. In the following year a class-action lawsuit was filed for the patients still alive and the families of the deceased. Then in 1974, a settlement of 10 million dollars was reached for those[10]. The United States government once again promised lifetime health benefits to those who were still living with the disease as well as burial service[11]. In 1975 it was expanded to cover loved ones of the effected in the study. The last study patient under the Tuskegee experiments died in 2004[11].
Transmission and Symptoms
There are multiple ways in which syphilis is transferred. The primary way is through the action of intercourse, in which there is bacterial transfer to another viable host[4]. The second most common is seen in utero from mother to baby (congenital syphilis) where the bacteria is able to go through the placenta and make contact with the fetus[2][4]. The last way is through accidental inoculation, in which there is some contact but not necessarily in a sexual manner. T. pallidum looks for a way to breach into cells for infection. T. pallidum’s course of action is able to penetrate dermal tissue that has abrasions, or through an intact mucous membrane. T. pallidum works by attaching to the host cell of interest by using adhesion starting the initial course of infection.
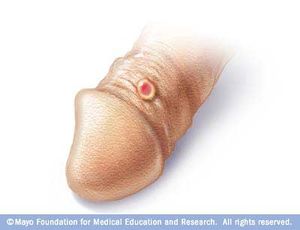
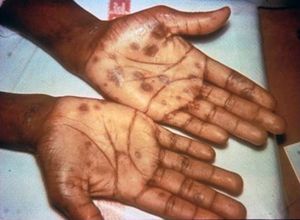
Syphilis has a total of four stages, which include primary syphilis, secondary syphilis, latent syphilis, and late syphilis. During the primary stage of syphilis, T. pallidum has entered the body. T. pallidum works by attaching to the host cell of interest by using adhesion. Eventually sores, or chancres on the genitals will form at the site in which the bacterium has entered the body. The sores and chancres are painless, firm, and usually round (Figure 3)[2]. The first symptoms can take between 10-90 days to show. The sores usually last between three to six weeks and then the body will begin to heal[2]. This will then cause a painless sore at the site in which T. pallidum entered the host. If untreated it will progress to the secondary phase. During the secondary phase, rough rashes and sores will start to develop on your body and even in the mouth. There is the possibility of developing spots on your hands and feet (Figure 4), along with an immune responses such as swelling of the lymph nodes, fever, a sore throat, hair loss, noticeable weight loss, body aches, and unusual fatigue[2]. If syphilis is still not treated it will progress to the latent phase. In the latent phase of syphilis, the symptoms experienced in in the primary and secondary stage will eventually go away. Syphilis is a tricky disease. There could be a large period of time for years in which you notice none of the symptoms, as they do not appear in the latent phase. In the late phase which is typically uncommon, syphilis can lead to a reduction in coordination of your body especially in muscular movements. In some cases people who have syphilis paired with HIV have experienced nervous system impairment such as paralysis, loss of eye-sight, or even the formation of mental diseases like dementia[2]. Syphilis has been known to shut down vital organs if the individual reaches the late stage of syphilis without proper treatment. In congenital syphilis, it has been documented that up to two-thirds of new-born babies display no signs of the disease. Sometimes still births, and premature births may occur[2]. However, for the children that survive, syphilis is known to physically show within the first two years of a child's life[2][14]. These symptoms include an abnormally large liver, rashes, and inflammation of lungs. Around 40% of children develop the late stages of syphilis, which can lead to saddle nose formation and other[14]. The major issues when it comes to diagnosing syphilis, is the traditional symptoms people develop are often similar to other diseases, which leads to improper treatment for the patient. In today’s world we have tests that readily allow us to detect syphilis. These include antibody test by drawing blood, a non-treponomal antibody test, a rapid plasma regain, the venereal disease research laboratory test, and physical examination.
Incidence
Most syphilis cases are usually seen in individuals in their late teens to early thirties. The incidence rate for syphilis is about 1 in every 3,885 people in the United States[12]. It has been estimated that nearly 70,000 intercourse related transmitted infections happen per year in the United States[12]. Worldwide incidence is around 12 million people annually. The cause is due to third world countries lacking sufficient resources and most importantly educating people. An estimation of deaths per year dating back to 1999 says on average, in the United States, 32 people die per year. Based on the World Health Organization statistics from 2002, mortality globally was around 155,000 people[12].
Research for a Cure and Conclusion
Around the 1900’s, Paul Erlich and colleagues developed a drug that utilized via chemotherapy. Salvarsan a potentially harmful drug was discovered to have some antimicrobial properties effective enough to kill the parasite the drugs name was Salvarsan. Prior to this era other harmful drugs were used such as mercury compounds along with numerous compounds, but they were never proven to be effective[9]. Just like mercury, Slavarsan had a downside. Salvarsan is an arsenical drug which could lead to a number of health related issues including death. The main issue with this drug was that it wasn’t target specific for the bacterium, it could easily attack the host which is what they wanted to avoid. Salvarsan was difficult to administer, and for it to be effective it required multiple treatments over a prolonged period of time[9]. John Mahoney, AD Harris, and Richard Arnold began research in the 1940’s on possible syphilis treatments, after reading a paper produced by Wallace Herrell, and colleagues that worked at the Mayo Clinic. Wallace Herrell’s paper described the effectiveness of penicillin against a strain of sulfonamide-resistant gonorrhea. Mahoney and his group of colleagues began a study at the US Marine Hospital in New York, where they actively treat four patients with primary stage of syphilis. The penicillin was administered by an intramuscular injection four times per hour, over an eight day period. Today, this same technique is used but only requires one injection of Benzathine penicillin G for people going through the primary, secondary or latent phase of the disease[15]. Penicillin has been used to cure patients with syphilis for over 70 years, surprisingly, syphilis is still very sensitive to this form of treatment as there have not been any reported signs of evolving T. pallidum to form any antibiotic resistant strain. Azithromycin is another drug of choice which can be used[15]. This version is taken orally as opposed to receiving shots of penicillin. The rabbit is the model organism of choice when it comes to research for a vaccine as they have a form of syphilis that is similar to the human form. Dr. James Miller in 1973, created an immunization protocol for a set of rabbits which received a total of 60 γ-irradiated shots of T. pallidum over a 37-week period, which was followed by intradermal Nichols strain T. pallidum. He observed that the rabbits that received these injections showed no signs of typical symptoms associated with syphilis at the injection sites. He also noticed there was no infection in naïve animals receiving lymph nodes from rabbits that had been immunized. The immunization process of the rabbits proved to be successful for up to a year after the final immunization received. The protective immunity against re-infection of the host showed that re-infection could occur homologs of T. pallidum, but the process of re-infection occurs at a slower rate in the animal model of the rabbit. This rather slower re-infection can be due to the rabbit’s tuned immune system that was treated heavily with the strain of T. pallidum. The immune system has some recognition of the bacterium slowing down the rate of infection. This outstanding research was ground-breaking as Dr. John Miller was able to find a temporary vaccination for the model species, but on the other hand, this method was not feasible for humans.
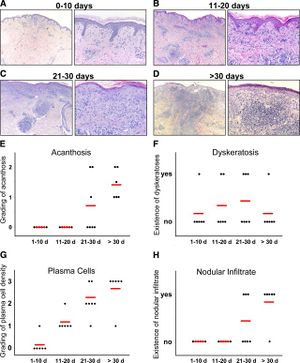
In a paper written by Stary et al., the group of researchers investigated the human immune system defense mechanism against secondary syphilis lesions.There were a total of 25 patients, 13 of which were HIV positive. Individuals with syphilis and HIV have a lower CD4+ T-cell count versus those with just syphilis. A biopsy was performed on each patient; these samples were classified based on the duration of the secondary syphilis symptoms (0-10 days, 11-20 days, 21-30 days, >30 days)[16]. Individuals with HIV had also had a higher count in the number of T. pallidum present. The innate immune system would respond sending macrophages, and neutrophils to sites of infection. They observed that the HIV negative group had CD4 and CD8 T-cells present while the HIV positives had only CD8 T-cells present(Figure 5). This is most likely due to having concurrent diseases. It was concluded that both CD4 and CD8 T-cells are important for combating syphilis. They then compared these biopsies graphically showing the differences in acanthosis, dykeratosis, plasma cells and nodular infiltrate of the differing time periods. There appeared to be a rather clear trend for acanthosis plasma cells nodular infiltrates; the longer the individual had secondary syphilis there a clear increase in all those three observable variables (Figure 5)[16]. The individuals were then clustered into 4 groups depending on how long they've displayed secondary symptoms.
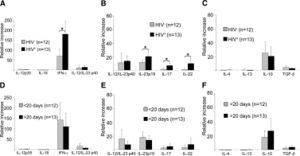
Stary et al., conducted more research to find the prevalence of cytokines in the biopsies using quantitative RT-PCR.IFN-y mRNA was in syphilitic skin was up-regulated in comparison to normal healthy skin (Figure 6)[16]. The quantitative RT-PCR revealed, HIV positive individuals with lesions, had a larger up-regulation compared to HIV negative individuals. This difference however, was not observed between lesions of different time stages. IFN-y, IL-23p19, IL-17, and IL-22 were all up regulated in HIV positive patients[16]. They concluded the T-cell producing cytokines for Th1 and Th17 in the secondary syphilis stage are vastly important for fighting the infection of T. pallidum leading to the amplified response[16].
Finding a cure has not been as simple as many researchers have tried and had moderate success. Being able to culture T. pallidum under lab conditions has proven to be difficult and cumbersome[2]. T. pallidum requires such unique conditions from temperature to pH and even levels of oxygen it can be exposed to. This difficulty exhibited in the lab makes it difficult to formulate a reliable vaccine[2]. Like most viruses we are immune to, they’ve been attenuated for use. As for T. pallidum if it does not have a mammalian host to infect, it is unable to carry out its life cycle making it difficult to observe in an artificial environment. Although T. pallidum is a nuissance it poses no true threat if caught early. There have also been no documented cases of mutated forms resistant to penicillin. However, it has been documented that the country of Cuba has eliminated the transmission of syphilis between mother and child [13].
References
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
1.Tampa M., et al., (2014). “Brief History of Syphilis“ Journal of Medicine and Life 7(1):4-10 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3956094/
2.LaFond R.E., Lukehart S., (2006). “Biological Basis for Syphilis” Clinical Microbiology Review 19(1):29-49 http://cmr.asm.org/content/19/1/29.full?view=long&pmid=16418521#sec-1
3.Souza de E.M.,(2005). “A hundred years ago, the discovery of Treponema pallidum” 80(5):547-8 http://www.scielo.br/pdf/abd/v80n5/en_v80n5a17.pdf
4.Cameron C.E., Lukehart S., (2014). “Current Status of Syphilis Vaccine Development: Need, Challenges, Prospects” Vaccine 32(14): 1602-1609 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3951677/
5.Schwartz W.F., (1961) Public Health Image Library http://phil.cdc.gov/phil/details.asp?pid=10179#modalIdString_CDCImage_0
7.Santis de M., et al.,(2012) “Syphilis infection during Pregnancy : Fetal Risks and Clinical Management” Infectious Diseases in Obstetrics and Gynecology Volume 2012: 5 pages http://www.hindawi.com/journals/idog/2012/430585/
8.Radolf J., Steiner B., Shevchenko D.,(1999). “Treponema Pallidum: doing a remarkable job with what its got” Trends in Microbiology vol. 7(1):7-9 http://www.sciencedirect.com/science/article/pii/S0966842X9801422X
9.Singh A., Ramonowski B., (1999). “Syphilis: Review with Emphasis on Clinical, Epidemiologic, and some Biologic Features ” Clinical Microbiology Review 12(2):187-209 http://cmr.asm.org/content/12/2/187.full https://www.researchgate.net/publication/16188760_The_Theoretical_Basis_of_Paul_Ehrlich's_Chemotherapy
10."The Tuskeegee Timeline" 19 February 2016. http://www.cdc.gov/tuskegee/timeline.htm
11.McCallum J.M., et al., (2006). “Awareness and Knowledge of the U.S. Public Health Service Syphilis Study at Tuskegee: Implications for Biomedical Research” Journal of Health Care Poor Underserved 17(4): 716-733 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1828138/
12."Deaths from Syphilis" 13 August 2015. http://www.rightdiagnosis.com/s/syphilis/deaths.htm
13."WHO validates elimination of mother-to-child transmission of HIV and syphilis in Cuba" 30 June 2015. http://www.who.int/mediacentre/news/releases/2015/mtct-hiv-cuba/en/
14.Woods R.C.,(2009). "Congenital Syphilis-Persisting Pestilence" Pediatric Infectious Disease Journal 28(6):536-7. http://journals.lww.com/pidj/Citation/2009/06000/Congenital_Syphilis_Persisting_Pestilence.16.aspx
15. "Syphilis Treatment and Care" 3 December 2015. http://www.cdc.gov/std/syphilis/treatment.htm
16. Stary R.C., et al., (2010). "Host Defense Mechanisms in Secondary Syphilitic Lesions : A Role for IFN-γ-/IL-17–Producing CD8+ T Cells?" The American Journal of Pathology 177(5):2421–2432 http://www.sciencedirect.com/science/article/pii/S0002944010602945
