The Lung Microbiome and its Correspondence with Asthma: Difference between revisions
(Created page with "==Introduction== The gut microbiome, an abundant microbial community that houses more than 1000 bacterial species<ref name="Loverdos">Loverdos, Konstantinos et al. “Lung Mi...") |
|||
| (41 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction:== | ||
[[Image:Huffnagle-Dickson-Lukacs.jpg|thumb|300px|right|"Comparison of the anatomy and bacterial microbiomes of the upper regions of the aerodigestive tract in humans. Bacterial composition is shown at the phylum-level. Gammaproteobacteria are a class in the Proteobacteria phylum."[https://www.nature.com/articles/mi2016108].]] | |||
In current research, the lung microbiome has begun to be linked to asthma, the most common place respiratory disease that is present in all ages, but most commonly begins in childhood, affecting each individual’s lower airways in varied frequency and severity. More than 250,000 deaths are linked to asthma every year.<ref name="Frati">Frati, Franco et al. “The Role of the Microbiome in Asthma: The Gut⁻Lung Axis.” International journal of molecular sciences vol. 20,1 123. 30 Dec. 2018, doi:10.3390/ijms20010123</ref> | |||
The gut microbiome, an abundant microbial community that houses more than 1000 bacterial species,<ref name="Loverdos">Loverdos, Konstantinos et al. “Lung Microbiome in Asthma: Current Perspectives.” Journal of clinical medicine vol. 8,11 1967. 14 Nov. 2019, doi:10.3390/jcm8111967</ref> is a highly researched and reviewed topic. However, in recent years, scientists have begun to study areas in the body where other microbiomes exist, such as lungs. Researchers have discovered that the <b>lung microbiome</b>, a microbial community in the lower respiratory tract, is 1) matured and 2) metabolically active. The lung microbiome in its entirety consists of its microorganisms (bacteria, archaea, eukaryotes, viruses), their genes, and the external environmental conditions of the lungs.<ref name="Loverdos" /> In comparison to other microbial communities, the lung microbiome is modest in size and provides some difficulties when attempting to retrieve samples. In regards to the lung microbiome's relation to <b>asthma</b>, the lung microbiome affects both the endotypes (subtypes of asthma) and phenotypes of asthma.<ref>Lötvall, Jan, et al. “Asthma Endotypes: A New Approach to Classification of Disease Entities within the Asthma Syndrome.” Journal of Allergy and Clinical Immunology, Mosby, 31 Jan. 2011, https://www.sciencedirect.com/science/article/pii/S0091674910018580?via%3Dihub.</ref> An infection of a host body is usually followed by an increase in gammaproteobacteria, which includes gram-negative bacteria that can resist certain antibiotics and releases molecules in the lungs that trigger <b>inflammation</b>. When the body experiences inflammation, it increases the amount of blood flow and mucus, which are nutrients for gammaproteobacteria. Therefore, gammaproteobacteria increases its reproduction as the environmental conditions of inflammation increase, causing a <b>cyclical inflammatory mechanism</b>. <ref name="Huffnagle">Huffnagle, G B, et al. “The Respiratory Tract Microbiome and Lung Inflammation: A Two-Way Street.” Nature News, Nature Publishing Group, 14 Dec. 2016, https://www.nature.com/articles/mi2016108.</ref> | |||
==Genetics of Asthma and the Microbes Located in the Lungs:== | |||
[[Image:Akhabir.gif|thumb|300px|right|"Genome-wide association study results for asthma and related traits. Associated phenotypes are indicated as follows:asthma in plain font, atopy underlined, total IgE in bold font, eosinophil count in italics, TDI-induced asthma in italics and underlined."[https://onlinelibrary.wiley.com/doi/10.1111/j.1440-1843.2011.01939.x#].]] | |||
== | ===Genes Linked to Asthma=== | ||
When considering the genetics behind microbes in the lung microbiome and its interactions with asthma, the genetics of asthma and the genetics of the microbes in the lung microbiome can be studied individually. Geneticists have found that asthma is not caused by a single mutation in a single gene and is a polygenic, <b>multifactorial disorder</b> impacted by both genetics and environmental factors, straying from the simple Mendelian inheritance. The risk of developing asthma depends on both the degree of genetic relatedness and the degree of severity of the relative’s asthma, meaning that the expression of the asthma phenotype is highly variable.<ref name="Thomsen">Thomsen, Simon F. “Genetics of asthma: an introduction for the clinician.” European clinical respiratory journal vol. 2 10.3402/ecrj.v2.24643. 16 Jan. 2015, doi:10.3402/ecrj.v2.24643</ref> In children with one asthma affected parent, the chance of recurrence is around 25%.<ref name="Thomsen" /> Individuals with asthma have a group of genes that set the “background risk” for asthma that is then acted upon by modifying genes and environmental elements. In order to identify these “background risk” genes and modifying genes, one can use genetic linkage analysis or genetic association analysis. Through genetic linkage analysis and genetic association analysis, geneticists have found over a hundred different genes linked to asthma, that usually fall into the following categories: function of the immune system, mucosal biology and function, or lung function and disease expression.<ref name="Thomsen" /> For example, the gene <b>ADAM33</b> was found to be connected to airway remodeling, disease progression, and chronic obstructive pulmonary disease while the protein <b>Filaggrin</b> was found to be connected to the maintenance of an effective skin barrier when located on chromosome 1q21. In a current study of experimental drug blocking on the IL-4 / IL-3 pathway, IL4 receptor amino acid variations were able to “predict” which patients would respond the best in terms of increased lung function to therapeutic treatments.<ref name="Slager">Slager, Rebecca E et al. “IL-4 receptor α polymorphisms are predictors of a pharmacogenetic response to a novel IL-4/IL-13 antagonist.” The Journal of allergy and clinical immunology vol. 126,4 (2010): 875-8. doi:10.1016/j.jaci.2010.08.001</ref> This study exemplifies that the future of asthma treatment lies in personalised medicine and pharmacogenetics. | |||
===Genetics of Microbes Present in the Lung Microbiome=== | |||
In terms of genetics and the lung microbiome, bacterial DNA was discovered in amniotic fluid, which scientists use as evidence that the lung microbiome formation is initiated in the fetus.<ref name="Loverdos" /> Some taxons of Gammaproteobacteria that are found in the lung microbiome have encoded into their genome the ability to use inflammatory byproducts to survive during low oxygen conditions, causing a <b>bacterial bloom</b> during asthma inflammation. Gammaproteobacteria also have the ability to promote inflammation encoded in their genome. MAMPS, also called <b>microbe-associated molecular patterns</b>, are on the surface of Gammaproteobacteria and interact with receptors to stimulate inflammation. This creates a feedback loop in which the bacteria that stimulates the most inflammation benefit from the effects of inflammation, as they feed off of the mucus created in an inflammation response. <ref name="Huffnagle" /> | |||
==Composition of the the Lung Microbiome:== | |||
[[Image:Goleva.png|thumb|300px|left|"The taxonomic composition of the airway microbiome in corticosteroid-resistant (CR) and corticosteroid-sensitive (CS) subjects with asthma with (bact. exp.) and without (no bact. exp.) bacterial expansions. . . Twenty-four subjects with CR asthma and nine subjects with CS asthma were found to have bacterial expansions as compared with normal control subjects; five CR subjects with asthma and one CS subject with asthma did not have bacterial expansions."[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3863730/].]] | |||
== | The lung microbiome is a low density community with a small bacterial population. A healthy lung microbiome consists of waves of immigration and elimination with Proteobacteria, Firmicutes, Bacteridetes, and Actinobacteria as the common phyla and Prevotella, Streptococcus, Veillonella, Neisseria, Haemophilus, and Fusobacteria as the common genera.<ref name ="Mathieu">Mathieu, Elliot et al. “Paradigms of Lung Microbiota Functions in Health and Disease, Particularly, in Asthma.” Frontiers in physiology vol. 9 1168. 21 Aug. 2018, doi:10.3389/fphys.2018.01168</ref> As the body develops a chronic lung disease like asthma, the contents of the lung microbiome change, switching from the phylum Bacteroidetes to Gammaproteobacteria.<ref name="Huffnagle" /> Defense against these changes includes “cough reflex mucociliary clearance, and innate and adaptive immunity.”<ref name="Loverdos" /> To study the microbiome of a patient, scientists use the process of <b>bronchoscopy</b> to retrieve samples, which causes some discrepancies in the data due to contamination of the sample when brought back up the throat. Studies show that as patients develop asthma, the lung microbiome shifts towards a more diverse species population. In a bronchoalveolar analysis of children with severe asthma, the most common phylum in the control (non-asthmatic) group was Prevotella whereas the most common phylum in the test (asthmatic) group was Staphylococcus and Haemophilus.<ref name="Mathieu" /> | ||
===Current Studies in Corticosteroid Resistant Asthma and the Lung Microbiome=== | |||
In a current study on the role of the lung microbiome in <b>corticosteroid response</b> in asthma, researchers attempted to find a new treatment strategy for asthma patients that are resistant to corticosteroids (a popular therapy to control airway inflammation in persistent asthma). 16S rRNA gene sequencing was performed on bronchoalveolar lavage samples of 29 subjects with corticosteroid resistant (CR) asthma, 10 subjects with corticosteroid sensitive (CS) asthma, and 12 control subjects. Their corticosteroid responsiveness was characterized, followed by the stimulation of their BAL macrophages with pathogenic and commensal microorganisms. Finally, the results were analyzed by PCR for the expression of corticosteroid regulated genes and cellularp38 mitogen-activated protein kinase (MAPK) activation.<ref name ="Goleva">Goleva, Elena et al. “The effects of airway microbiome on corticosteroid responsiveness in asthma.” American journal of respiratory and critical care medicine vol. 188,10 (2013): 1193-201. doi:10.1164/rccm.201304-0775OC</ref> | |||
=====Results of CR Asthma and CS Asthma Study===== | |||
The following comparisons between the results from CR patients and CS patients to control patients showed significant changes in the lung microbiome composition that included expansion in the Prevotella and Veillonella genera, reduction in the Fusobacteria genera, and expansion in the Actinobacteria and Proteobacteria phylum. CS patients and CR patients microbiome only differed at genus level, showing the CR patients with an expansion of gram-negative LPS (lipopolysaccharide) producing bacteria with “short acyl chains lipid A LPS structures with known high endotoxic activity”<ref name="Goleva" /> like Haemophilus parainfluenzae. These bacteria trigger TAK1 / MAPK activation and induce corticosteroid resistance. When TAK1 is inhibited, corticosteroid sensitivity is restored. Therefore, future studies should explore TAK1 inhibition as a potential therapy for patients with CR asthma. | |||
==Conclusion== | |||
The lung microbiome, once believed to be a barren microbial community, is surfacing as an important environment for bacteria that affects lung respiratory diseases like asthma. Looking into the specifics of the lung microbiome can help scientists create treatments for asthmatic patients that are unresponsive to the common treatments for asthma, like patients with corticosteroid resistant asthma. Asthma is a highly variable disease, meaning that scientists will continue to discover more genes and outside factors that contribute to its development in a patient. The lung microbiome is relatively newly discovered factor that will continue to shed insights on how asthma develops in severity and frequency (of asthma attacks). As technology develops further, we will hopefully discover a more efficient way to take samples of the lung microbiome, other than a bronchoscopy, that is contamination free so that data is more accurate in future studies. | |||
==References== | ==References== | ||
Latest revision as of 15:06, 8 December 2021
Introduction:
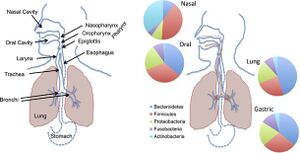
In current research, the lung microbiome has begun to be linked to asthma, the most common place respiratory disease that is present in all ages, but most commonly begins in childhood, affecting each individual’s lower airways in varied frequency and severity. More than 250,000 deaths are linked to asthma every year.[1] The gut microbiome, an abundant microbial community that houses more than 1000 bacterial species,[2] is a highly researched and reviewed topic. However, in recent years, scientists have begun to study areas in the body where other microbiomes exist, such as lungs. Researchers have discovered that the lung microbiome, a microbial community in the lower respiratory tract, is 1) matured and 2) metabolically active. The lung microbiome in its entirety consists of its microorganisms (bacteria, archaea, eukaryotes, viruses), their genes, and the external environmental conditions of the lungs.[2] In comparison to other microbial communities, the lung microbiome is modest in size and provides some difficulties when attempting to retrieve samples. In regards to the lung microbiome's relation to asthma, the lung microbiome affects both the endotypes (subtypes of asthma) and phenotypes of asthma.[3] An infection of a host body is usually followed by an increase in gammaproteobacteria, which includes gram-negative bacteria that can resist certain antibiotics and releases molecules in the lungs that trigger inflammation. When the body experiences inflammation, it increases the amount of blood flow and mucus, which are nutrients for gammaproteobacteria. Therefore, gammaproteobacteria increases its reproduction as the environmental conditions of inflammation increase, causing a cyclical inflammatory mechanism. [4]
Genetics of Asthma and the Microbes Located in the Lungs:
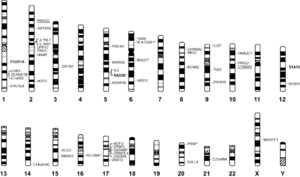
Genes Linked to Asthma
When considering the genetics behind microbes in the lung microbiome and its interactions with asthma, the genetics of asthma and the genetics of the microbes in the lung microbiome can be studied individually. Geneticists have found that asthma is not caused by a single mutation in a single gene and is a polygenic, multifactorial disorder impacted by both genetics and environmental factors, straying from the simple Mendelian inheritance. The risk of developing asthma depends on both the degree of genetic relatedness and the degree of severity of the relative’s asthma, meaning that the expression of the asthma phenotype is highly variable.[5] In children with one asthma affected parent, the chance of recurrence is around 25%.[5] Individuals with asthma have a group of genes that set the “background risk” for asthma that is then acted upon by modifying genes and environmental elements. In order to identify these “background risk” genes and modifying genes, one can use genetic linkage analysis or genetic association analysis. Through genetic linkage analysis and genetic association analysis, geneticists have found over a hundred different genes linked to asthma, that usually fall into the following categories: function of the immune system, mucosal biology and function, or lung function and disease expression.[5] For example, the gene ADAM33 was found to be connected to airway remodeling, disease progression, and chronic obstructive pulmonary disease while the protein Filaggrin was found to be connected to the maintenance of an effective skin barrier when located on chromosome 1q21. In a current study of experimental drug blocking on the IL-4 / IL-3 pathway, IL4 receptor amino acid variations were able to “predict” which patients would respond the best in terms of increased lung function to therapeutic treatments.[6] This study exemplifies that the future of asthma treatment lies in personalised medicine and pharmacogenetics.
Genetics of Microbes Present in the Lung Microbiome
In terms of genetics and the lung microbiome, bacterial DNA was discovered in amniotic fluid, which scientists use as evidence that the lung microbiome formation is initiated in the fetus.[2] Some taxons of Gammaproteobacteria that are found in the lung microbiome have encoded into their genome the ability to use inflammatory byproducts to survive during low oxygen conditions, causing a bacterial bloom during asthma inflammation. Gammaproteobacteria also have the ability to promote inflammation encoded in their genome. MAMPS, also called microbe-associated molecular patterns, are on the surface of Gammaproteobacteria and interact with receptors to stimulate inflammation. This creates a feedback loop in which the bacteria that stimulates the most inflammation benefit from the effects of inflammation, as they feed off of the mucus created in an inflammation response. [4]
Composition of the the Lung Microbiome:

The lung microbiome is a low density community with a small bacterial population. A healthy lung microbiome consists of waves of immigration and elimination with Proteobacteria, Firmicutes, Bacteridetes, and Actinobacteria as the common phyla and Prevotella, Streptococcus, Veillonella, Neisseria, Haemophilus, and Fusobacteria as the common genera.[7] As the body develops a chronic lung disease like asthma, the contents of the lung microbiome change, switching from the phylum Bacteroidetes to Gammaproteobacteria.[4] Defense against these changes includes “cough reflex mucociliary clearance, and innate and adaptive immunity.”[2] To study the microbiome of a patient, scientists use the process of bronchoscopy to retrieve samples, which causes some discrepancies in the data due to contamination of the sample when brought back up the throat. Studies show that as patients develop asthma, the lung microbiome shifts towards a more diverse species population. In a bronchoalveolar analysis of children with severe asthma, the most common phylum in the control (non-asthmatic) group was Prevotella whereas the most common phylum in the test (asthmatic) group was Staphylococcus and Haemophilus.[7]
Current Studies in Corticosteroid Resistant Asthma and the Lung Microbiome
In a current study on the role of the lung microbiome in corticosteroid response in asthma, researchers attempted to find a new treatment strategy for asthma patients that are resistant to corticosteroids (a popular therapy to control airway inflammation in persistent asthma). 16S rRNA gene sequencing was performed on bronchoalveolar lavage samples of 29 subjects with corticosteroid resistant (CR) asthma, 10 subjects with corticosteroid sensitive (CS) asthma, and 12 control subjects. Their corticosteroid responsiveness was characterized, followed by the stimulation of their BAL macrophages with pathogenic and commensal microorganisms. Finally, the results were analyzed by PCR for the expression of corticosteroid regulated genes and cellularp38 mitogen-activated protein kinase (MAPK) activation.[8]
Results of CR Asthma and CS Asthma Study
The following comparisons between the results from CR patients and CS patients to control patients showed significant changes in the lung microbiome composition that included expansion in the Prevotella and Veillonella genera, reduction in the Fusobacteria genera, and expansion in the Actinobacteria and Proteobacteria phylum. CS patients and CR patients microbiome only differed at genus level, showing the CR patients with an expansion of gram-negative LPS (lipopolysaccharide) producing bacteria with “short acyl chains lipid A LPS structures with known high endotoxic activity”[8] like Haemophilus parainfluenzae. These bacteria trigger TAK1 / MAPK activation and induce corticosteroid resistance. When TAK1 is inhibited, corticosteroid sensitivity is restored. Therefore, future studies should explore TAK1 inhibition as a potential therapy for patients with CR asthma.
Conclusion
The lung microbiome, once believed to be a barren microbial community, is surfacing as an important environment for bacteria that affects lung respiratory diseases like asthma. Looking into the specifics of the lung microbiome can help scientists create treatments for asthmatic patients that are unresponsive to the common treatments for asthma, like patients with corticosteroid resistant asthma. Asthma is a highly variable disease, meaning that scientists will continue to discover more genes and outside factors that contribute to its development in a patient. The lung microbiome is relatively newly discovered factor that will continue to shed insights on how asthma develops in severity and frequency (of asthma attacks). As technology develops further, we will hopefully discover a more efficient way to take samples of the lung microbiome, other than a bronchoscopy, that is contamination free so that data is more accurate in future studies.
References
- ↑ Frati, Franco et al. “The Role of the Microbiome in Asthma: The Gut⁻Lung Axis.” International journal of molecular sciences vol. 20,1 123. 30 Dec. 2018, doi:10.3390/ijms20010123
- ↑ 2.0 2.1 2.2 2.3 Loverdos, Konstantinos et al. “Lung Microbiome in Asthma: Current Perspectives.” Journal of clinical medicine vol. 8,11 1967. 14 Nov. 2019, doi:10.3390/jcm8111967
- ↑ Lötvall, Jan, et al. “Asthma Endotypes: A New Approach to Classification of Disease Entities within the Asthma Syndrome.” Journal of Allergy and Clinical Immunology, Mosby, 31 Jan. 2011, https://www.sciencedirect.com/science/article/pii/S0091674910018580?via%3Dihub.
- ↑ 4.0 4.1 4.2 Huffnagle, G B, et al. “The Respiratory Tract Microbiome and Lung Inflammation: A Two-Way Street.” Nature News, Nature Publishing Group, 14 Dec. 2016, https://www.nature.com/articles/mi2016108.
- ↑ 5.0 5.1 5.2 Thomsen, Simon F. “Genetics of asthma: an introduction for the clinician.” European clinical respiratory journal vol. 2 10.3402/ecrj.v2.24643. 16 Jan. 2015, doi:10.3402/ecrj.v2.24643
- ↑ Slager, Rebecca E et al. “IL-4 receptor α polymorphisms are predictors of a pharmacogenetic response to a novel IL-4/IL-13 antagonist.” The Journal of allergy and clinical immunology vol. 126,4 (2010): 875-8. doi:10.1016/j.jaci.2010.08.001
- ↑ 7.0 7.1 Mathieu, Elliot et al. “Paradigms of Lung Microbiota Functions in Health and Disease, Particularly, in Asthma.” Frontiers in physiology vol. 9 1168. 21 Aug. 2018, doi:10.3389/fphys.2018.01168
- ↑ 8.0 8.1 Goleva, Elena et al. “The effects of airway microbiome on corticosteroid responsiveness in asthma.” American journal of respiratory and critical care medicine vol. 188,10 (2013): 1193-201. doi:10.1164/rccm.201304-0775OC
Edited by Margo Moceyunas, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2020, Kenyon College.
