The Mycorrhizal Network
By Freya Beinart
Introduction

Mycorrhizae is the symbiotic relationship between fungi and plants. Mycelium, the vegetative part of a fungus, extends in a branching network of hyphae that secrete enzymes, breaking down organic material into nutrients. The vast mycelial branches of the fungi are made of branching networks of hyphae which, in a mycorrhizal association, colonize the root systems of many plant species, greatly increasing the surface area of a plant’s rhizosphere in which it collects water and essential nutrients. In exchange for these nutrients, the plants provide the fungi with carbohydrates that the fungi cannot produce on its own, being heterotrophic. The hyphae of soil fungi colonize the root system of plants, providing the photosynthetic organisms with nutrients and water in exchange for carbohydrates that the heterotrophic fungi cannot produce on its own. This colonization may be intracellular as arbuscular mycorrhizal fungi (AMF) which trade nutrients via a signal transduction pathway, or extracellular as ectomycorrhizal fungi that envelop plant roots.
Types of Mycorrhizal Associations
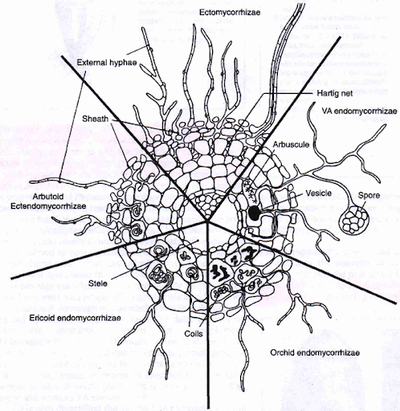
Characterization of mycorrhizal association is dependent upon the location of fungal colonization and is specific to both the plant species and their fungal symbionts.
Endomycorrhizae
Endomycorrhizal symbioses occur when the mycorrhizal fungi colonizes a host plant intracellularly.
Arbuscular mycorrhizal fungi
Fungi of the phylum Glomeromycota that forms mutualistic symbioses, called arbuscular mycorrhizae, with vascular plants are referred to as arbuscular mycorrhizal fungi, or AMF. These fungi are associated with 90% of vascular plant species. AM fungi are unable to live without the presence of the host plants, as they depend on the transfer of carbohydrates from the plant tissue, making the fungi obligate biotrophs [1]. Plant taxa that are observed to exhibit this relationship are bryophyta, pteridophyta, gymnosperms, and angiosperms [2]
.
Hyphae from a germinating spore infect the host root, passing the epidermis and penetrating cortical cells which form arbuscules - the structure from which the name of these fungi originated [3]. The arbuscules are networks of extremely fine clustered hyphae within the host cells where nutrient transfer is centralized [4].
The hyphae may also form vesicles between or within the root cells, acting as an organ for storage with a thickened cell wall which aids in the establishment of new colonies [5] [6]. The mycelium which extends from the plant root is called the extraradical mycelium, which connects from plant to plant, forming a continuum of nutrient and water exchange [7].
Ectomycorrhizal fungi
Ericoid mycorrhizae
Evolution of Mycorrhizae
Fossil records indicate that mycorrhizal fungi predates the evolution of vascular plants, about 460 million years ago in the Ordovican period [8]. The most ancestral mycorrhizal fungi has been identified as arbuscular, penetrating the root cortical cells in most extant plant taxa. The phylum of fungi that associates with land plants as arbuscular mycorrhizal fungi (AMF) is that of Glomeromycota [9] The presence of these fungi were involved in the development of soil and the evolution and colonization of vascular land plants, specifically by aiding the non-vascular plants with acquisition of nutrients through fungal hyphae, as the “soil” lacked a significant amount of nutrients for terrestrial plants to survive without their fungal symbionts [10]. As the fungi cycles nutrients such as nitrogen, phosphorus, and sulfur to the plants, the fungus receives and fixes carbon into the ground, assisting in the lowering of atmospheric CO2 leading to the oxygenation of the atmosphere during the development of terrestrial plants [11] [12]. In a study by Krings et al (2007) fossil evidence from the Rhynie chert sediment of the Early Devonian period suggests that fungal endophytes, specifically those which colonize the rhizoids of Nothia aphylla, actively influenced the evolution of these plants due to observed host responses. The responses to fungal infection in N. aphylla - rhizoid bulging, separation of infected cells via thickening of cell walls, and the motile inhibition of hypha - suggests its susceptibility to colonization by fungi at least 400 million years ago, advancing selection between plant species with the increasing complexity of interspecies interactions [13].
Biogeochemical Cycles
Nitrogen Fixation
Nitrogen is an extremely important nutrient for all living organisms as it is essential for amino acid synthesis and therefore the formation of proteins. Despite its importance, nitrogen cannot be used by many organisms until it has been heavily reduced into ammonia [14]. This anabolic process is very energy-intensive and therefore many organisms do not possess the ability to fix nitrogen (https://www.semanticscholar.org/paper/The-microbial-nitrogen-cycling-network-Kuypers-Marchant/ec5210fe0ef4ec95865bbd684127bf77abd782f1) (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC98982/). Organisms that can fix atmospheric nitrogen are known as diazotrophs - bacteria and archaea that do not require fixed nitrogen for their growth or survival (Takai 2019).
Root nodule symbiosis between plants and nitrogen-fixing bacteria is apparent in plants of the Fabaceae, also known as the legume family [15]. Legumes have a symbiotic relationship with rhizobia, single-celled, Gram-negative bacteria [16] [17]. The mutualism between the legumes and rhizobia allows the bacteria to take up atmospheric nitrogen, feeding it to the plant. In return, the plant provides carbohydrates to the bacteria as it is essential for their metabolic processes and growth (Zahran 1999). Although mycorrhizal symbionts do not possess the metabolism required for nitrogen fixation, studies have shown that mycorrhizae may indirectly affect nitrogen fixation as they increase root nodulation [18] [19] [20].
The legume symbiotic signaling pathway (SYM) has been studied in relation to arbuscular mycorrhizal symbiosis [21] [22]. The SYM pathway controls both nodulation of legumes and the mycorrhization of land plants, and is suggested to evolve first in legumes and adapted for arbuscular mycorrhizal symbiosis (https://gtr.ukri.org/projects?ref=BB%2FR017859%2F1#:~:text=A%20common%20SYM%20pathway%20controls,symbiosis%20(~400%20MYA)%20pathway.). This pathway is characterized by its use of calcium as a second messenger, regulating both AM and rhizobial symbiosis. The pathway consists of seven proteins, 5 which are required for Ca2+ spiking and 2 which are downstream of calcium spiking that transduce the signals.
Applications
Section 1
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
Section 2
Section 3
Section 4
Conclusion
References
- ↑ Smith, S. E., & Reads, D. J. (n.d.). Mycorrhizal Symbiosis. Retrieved April 06, 2021, from https://books.google.com/books?hl=en&lr=&id=qLciOJaG0C4C&oi=fnd&pg=PP1&ots=zrsXoTRxkN&sig=G4cYDH7aK0uYRBaENI2m3JvclNA#v=onepage&q&f=false
- ↑ Barman, J., Samanta, A., Saha, B., & Datta, S. (2016). Mycorrhiza. Resonance, 21(12), 1093-1104. doi:10.1007/s12045-016-0421-6
- ↑ Smith, S. E., & Reads, D. J. (n.d.). Mycorrhizal Symbiosis. Retrieved April 06, 2021, from https://books.google.com/books?hl=en&lr=&id=qLciOJaG0C4C&oi=fnd&pg=PP1&ots=zrsXoTRxkN&sig=G4cYDH7aK0uYRBaENI2m3JvclNA#v=onepage&q&f=false
- ↑ Moore, D., Robson, G., & Trinci, A. (2011, July 14). 21St century guidebook TO Fungi: Plant science. Retrieved April 05, 2021, from https://www.cambridge.org/gb/academic/subjects/life-sciences/plant-science/21st-century-guidebook-fungi?format=WW&isbn=9780521186957
- ↑ Brundrett, M. (1991). Mycorrhizas in natural ecosystems. In Advances in Ecological Research: Vol. 21 (pp. 171–313). Elsevier. doi: 10.1016/S0065-2504(08)60099-9
- ↑ Müller, A., Ngwene, B., Peiter, E., & George, E. (2017). Quantity and distribution of arbuscular mycorrhizal fungal storage organs within dead roots. Mycorrhiza, 27(3), 201–210. doi: 10.1007/s00572-016-0741-0
- ↑ Bhargava, P., Vats, S., & Gupta, N. (2019). Metagenomics as a tool to explore mycorrhizal fungal communities. Mycorrhizosphere and Pedogenesis, 207-219. doi:10.1007/978-981-13-6480-8_13
- ↑ Redecker, D., Kodner, R., & Graham, L. E. (2000). Glomalean fungi from the Ordovician. Science, 289(5486), 1920–1921. doi: 10.1126/science.289.5486.1920
- ↑ Schüβler, A., Schwarzott, D., & Walker, C. (2001). A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research, 105(12), 1413–1421. doi: 10.1017/S0953756201005196
- ↑ Pirozynski, K. A., & Malloch, D. W. (1975). The origin of land plants: a matter of mycotrophism. Bio Systems, 6(3), 153–164. doi: 10.1016/0303-2647(75)90023-4
- ↑ Mills, B. J. W., Batterman, S. A., & Field, K. J. (2018). Nutrient acquisition by symbiotic fungi governs Palaeozoic climate transition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 373(1739). doi: 10.1098/rstb.2016.0503
- ↑ Strullu-Derrien, C., Selosse, M.-A., Kenrick, P., & Martin, F. M. (2018). The origin and evolution of mycorrhizal symbioses: from palaeomycology to phylogenomics. The New Phytologist, 220(4), 1012–1030. doi: 10.1111/nph.15076
- ↑ Krings, M., Taylor, T. N., Hass, H., Kerp, H., Dotzler, N., & Hermsen, E. J. (2007). Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. The New Phytologist, 174(3), 648–657. doi: 10.1111/j.1469-8137.2007.02008.x
- ↑ Society, M. (n.d.). Nitrogen cycle: Microbes and the outdoors. Retrieved April 06, 2021, from https://microbiologysociety.org/why-microbiology-matters/what-is-microbiology/microbes-and-the-outdoors/nitrogen-cycle.html#:~:text=Nitrogen%20is%20required%20by%20all,and%20other%20nitrogen%20containing%20compounds.&text=It%20cannot%20be%20used%20in,with%20hydrogen)%2C%20to%20ammonia.
- ↑ Brewin, N. J. (2001). Root Nodules (Legume- Rhizobium Symbiosis). In John Wiley & Sons, Ltd (Ed.), Encyclopedia of life sciences. Chichester, UK: John Wiley & Sons, Ltd. doi: 10.1002/9780470015902.a0003720.pub2
- ↑ Brewin, N. J. (2001). Root Nodules (Legume- Rhizobium Symbiosis). In John Wiley & Sons, Ltd (Ed.), Encyclopedia of life sciences. Chichester, UK: John Wiley & Sons, Ltd. doi: 10.1002/9780470015902.a0003720.pub2
- ↑ Hayman, D. S. (1986). Mycorrhizae of nitrogen-fixing legumes. MIRCEN Journal of Applied Microbiology and Biotechnology, 2(1), 121–145. doi: 10.1007/BF00937189
- ↑ Brewin, N. J. (2001). Root Nodules (Legume- Rhizobium Symbiosis). In John Wiley & Sons, Ltd (Ed.), Encyclopedia of life sciences. Chichester, UK: John Wiley & Sons, Ltd. doi: 10.1002/9780470015902.a0003720.pub2
- ↑ Hayman, D. S. (1986). Mycorrhizae of nitrogen-fixing legumes. MIRCEN Journal of Applied Microbiology and Biotechnology, 2(1), 121–145. doi: 10.1007/BF00937189
- ↑ Mus, F., Crook, M. B., Garcia, K., Garcia Costas, A., Geddes, B. A., Kouri, E. D., … Peters, J. W. (2016). Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Applied and Environmental Microbiology, 82(13), 3698–3710. doi: 10.1128/AEM.01055-16
- ↑ Gutjahr, C., Banba, M., Croset, V., An, K., Miyao, A., An, G., … Paszkowski, U. (2008). Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. The Plant Cell, 20(11), 2989–3005. doi: 10.1105/tpc.108.062414
- ↑ Mus, F., Crook, M. B., Garcia, K., Garcia Costas, A., Geddes, B. A., Kouri, E. D., … Peters, J. W. (2016). Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Applied and Environmental Microbiology, 82(13), 3698–3710. doi: 10.1128/AEM.01055-16
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2021, Kenyon College.
