The Role of Clostridium perfringens Toxins in Gas Gangrene
What is Gas Gangrene and What Causes It?
By Marysol Arce
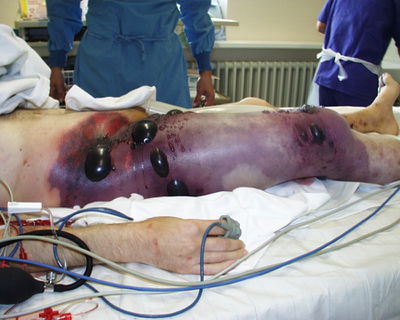
Gas gangrene is caused by a rod shaped, gram positive, spore forming bacterium called Clostridium perfringens. The bacterium lives in anaerobic environments like the soil and inside the gut of humans and animals (Williamson and Titball 1993). Clostridium perfringens is currently known to have five different strains, marked as: A, B, C, D, and E (Sakurai et al. 2004). The different strains are classified based on which toxins they produce: alpha toxin, beta toxin, epsilon toxin, and/or iota toxins (Shimizu et al. 2001). The Clostridium perfringens A strain is of the most importance because it causes gas gangrene, or myonecrosis, in humans. Gangrene is the death of body tissue and usually seen in elderly people or diabetic people. Open wounds or trauma into deep tissue that become infected by bacteria also become gangrenous. Gas gangrene is an especially dangerous form because it spreads quickly throughout the body if left untreated, and can be fatal. C. perfringens produces toxins that kills cells and releases gas, leaving parts of the body black, purple, and covered with blisters. The A strain of the C. perfringens bacterium produces two important toxins that enable the spread of the bacterium and tissue death: the alpha toxin and perfringolysin O (O’Brien and Melville 2004). The alpha toxin, also known as CPA or PLC, is a crucial virulence factor for this bacterium because gas gangrene cannot occur without it. However, the alpha toxin and perfringolysin O (PFO) work synergistically to accomplish the spread of infection (Awad et al. 2001). The toxins can cause septic shock (when infection leads to very low blood pressure) and hypoxia (reduced amounts of oxygen to body tissues). It even causes organ failure and muscle degeneration due to the lack of oxygen and reduced blood flow. The degeneration of the muscle and tissue releases gas that builds up under the skin and makes the area blister and bubble. The appearance of these blisters in various parts of the body usually indicates that the infection has progressed very far and that the tissue is decomposing. The body’s immune system is also rendered helpless because the toxins make the leukocytes clump together and clog in the blood vessels (leukostasis), unabling them to get to the site of infection (Stevens and Bryant 2002). The toxins also cause further blood vessel dysfunction, like vascular leakage which is what makes swelling a big characteristic of this disease.
Clostridium perfringens genome
The bacterium Clostridium perfringens’ genome is a lone circular chromosome with additional plasmids that are extra-chromosomal, meaning that there is other DNA found outside of the nucleus (Verherstraeten et al. 2015). It is made up of 2,660 regions that encode proteins and 10 ribosomal RNA genes (Shimizu et al. 2002). The genome also possesses the anaerobic fermentation enzymes usually found in bacterium that allow for gas production. However, it does not have any tricarboxylic acid (TCA) cycle enzymes or for a respiration mechanism. Many saccharolytic enzymes are present but most of the enzymes needed in amino acid biosynthesis are not present in the genome. There are at least 20 genes that encode virulence factors for the bacterium and 5 hyaluronidase genes, which encode enzymes that degrade hyaluronic acid, that also add to C. perfringens virulence (Shimizu et al 2001). There are also 4 regulons that regulate the pathogenicity, the ability of the organism to cause disease, of the bacterium. Clostridium perfringens utilizes and gains many different crucial materials needed to perform their function from the host cells by producing many degradative toxins and enzymes. To obtain what they need, the bacterium uses these toxins and enzymes to destroy the host’s tissues and cells. The two critical toxins of C. perfringens A strain, alpha toxin and perfringolysin O, are both chromosomally encoded (Li et al. 2013).
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
[1]
Alpha Toxin (PLC)
All strains of C. perfringens can produce the alpha toxin, also known as Clostridium perfringens alpha toxin (CPA) or by its main enzyme, phospholipase C (PLC) (Li et al. 2013). The alpha toxin is the most toxic enzyme that is produced by the A strain, and is also a crucial virulence factor. It has phospholipase C and sphingomyelinase that hydrolyzes phosphatidylcholine (PC) and sphingomyelin (SM), two very important components of a eukaryotic cell membrane (Awad et al. 2001). Alpha toxin degrades PC and SM in membranes and disrupts membranes, leading to cell lysis. For the alpha toxin to break down the host cell’s membrane, it promotes carboxyfluorescein (CF) leakage and phosphorylcholine release from the host’s liposomes which are made up of either PC or SM (Sakurai et al. 2004). By cleaving molecules from the exterior of the host cell's phospholipid bilayers and interrupting the membrane’s functions, it then promotes cell lysis and ultimately death. The alpha toxin’s membrane-damaging ability is also dependent of the membrane’s fluidity. Other actions caused by the toxin are hemolysis (rupture of red blood cells), dermonecrosis (death of skin tissue due to lack of blood flow), and lethality (Sakurai et al. 2004). The alpha toxin promotes endothelial cells (cells that line the inside of blood vessels) to be produced by intercellular mediators. In addition, it also promotes the production of the intercellular adhesion molecule 1, an endothelial leukocyte adhesion model, and a platelet activating factor (Sakurai et al. 2004). The toxin induces the relocation of the platelet fibrinogen receptor from the inside of the platelet to its membrane. This relocation then triggers the formation of more platelets and platelet aggregates (Sakurai et al. 2004). These actions contribute greatly to an elevated vascular permeability and leads to edema, defined by the buildup of fluid in the tissues. Essentially, the alpha toxin is able to escape the leukocytes and move out of the blood vessels and into the cells. The leukocytes are unable to move or stop the infection because they are stuck with the platelets and can only watch while the toxin kills the tissues. Researchers have also discovered that the activation of the sphingomyelin (SM) cycle is a fundamental event within the signal transduction pathway that takes part in cellular proliferation, differentiation, and apoptosis (Sakurai et al. 2004). A crystallography of alpha toxin reveals its structure to be composed of two domains: the N domain and the C domain. The N domain is composed of 9 ⍺-helices that are tightly packed together. The C domain consists of an 8 stranded antiparallel β-sandwich motif (Sakurai et al. 2004). The N domain also includes 3 divalent cations that contain zinc ions in the active site (Sakurai et al. 2004). Zinc is highly important for the alpha toxin because it is essential for the toxin’s activity in vivo and has also been found to enhance alpha toxin production in vitro (Stevens and Bryant 2002). Amino acid residues participate in zinc coordination and prove necessary for the toxin’s enzymatic activities (Sakurai et al. 2004). The C domain of alpha toxin also performs the most important part when lysing the host cells. The C domain is the part of the alpha toxin that is inserted into the host cell’s phospholipid bilayer to disrupt the membrane (Sakurai et al. 2004). The two domains are also highly crucial in hemolysis, the destroying of erythrocytes, or red blood cells. The N domain itself cannot bind or lyse the them; the C domain must be the one to bind to the red blood cell’s membrane (Nagahama et al. 2002). Then by combining the N domain and C domain, the alpha toxin gets it hemolytic power. To summarize, the alpha toxin elicits biological activities and processes that are inherent to tissues and cells. First, the toxin attaches to the binding site on the exterior of the membrane, then the C domain inserts itself into the host membrane. Next, the N domain follows: attacking and hydrolyzing the PC and SM within the membrane. The hydrolysis stimulates the signal transduction pathway through activation of phospholipase C and / or SMase (Sakurai et al. 2004).
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
All strains of C. perfringens produce the alpha toxin, also called CPA or PLC. The alpha toxin cleaves molecules from the surface the host cell's’ phospholipid bilayers and disrupts the membrane’s functions, which then promotes cell lysis and death.

Perfringolysin O (PFO)
All strains of C. perfringens can produce the toxin perfringolysin O (PFO). The structure of PFO is a signal peptide that is secreted by the general secretory pathway (GSP) in the bacterium. When this signal peptide passes through the cell membrane, it is cleaved, resulting in an water-soluble monomer in the extracellular space. This toxin is part of a pore-forming toxin family, the cholesterol-dependent cytolysin (CDC) family (Verherstraeten et al. 2015). The PFO that was secreted as the water-soluble monomer can only recognize and bind to cholesterol in the plasma membrane (Verherstraeten et al. 2015). Cholesterol is common component of animal cells and is required for the toxin to perform its function. Once recognizing and attaching to cholesterol in the membrane, PFO oligomerizes at the host cell’s exterior and constructs a pore complex. Next it inserts itself into the cell membrane to form a large pore (Li et al. 2013). The formation of this large pore disrupts the cell membrane, which causes cell lysis and cell death. But has been thought that cell lysis is not the main biological effect of this toxin on C. perfringens affected tissue (Li et al. 2013). PFO, as well as the alpha toxin, prevent white blood cell from arriving at the site of infection. Because the body’s immune system is not able to fight the bacterium at the site of infection, the toxin aids in the decay of flesh and tissue. Although the alpha toxin is critical to the virulence, PFO is not. Even though it may not be essential, it is synergistic with the alpha toxin. Both act together and boost the effect of the disease to speed up necrosis. If the host’s cells are lysed it makes it easier for the bacterium to infiltrate the cell and speeds up the process of necrosis. PFO is also like a double edged sword: it has a separate function in different stages of gas gangrene infection. In the early stages of infection, it causes macrophage cytotoxicity (O’Brien and Melville 2004). In the later stages of infection, it causes thrombosis, which is the clotting of blood cells in the blood vessels (Awad et al. 2001). Although PFO plays a big role in thrombosis, the alpha toxin does as well, and the two work synergistically (described further in the section: Synergy of Alpha Toxin and Perfringolysin O to Spread Gas Gangrene). To summarize PFO’s mechanism in cell lysis and furthering the infection, there are four main steps. First, the toxin binds to the cholesterol inside membranes. Second, it inserts itself into the membrane. Third, it oligomerizes, and fourth, it forms a pore that disrupts the cell (Shimada et al. 1999).
Include some current research, with at least one figure showing data.
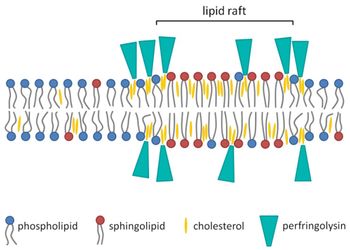
How Do These Toxins Help C. perfringens Persist and Spread in the Body?
Include some current research, with at least one figure showing data.
