The Role of Viral Proteins in Epstein-Barr Virus Induced Disease: Difference between revisions
No edit summary |
|||
| Line 20: | Line 20: | ||
While latent activity typically is the primary contributor to the development of lymphoproliferative diesease (LPD) in EBV infected individuals, lytic-incompetent EBV strains are less effective generators of LPD associated lesioning than the wild-type [11]. Lytic infection results in the secretion of paracrine factors that may stimulate the growth of latently infected B-cell lines [9, 11]. While it may have a more diffuse effect than the latent mechanism, EBV lytic infection mediated signalling may contribute to the development of lymphoproliferative disease. | While latent activity typically is the primary contributor to the development of lymphoproliferative diesease (LPD) in EBV infected individuals, lytic-incompetent EBV strains are less effective generators of LPD associated lesioning than the wild-type [11]. Lytic infection results in the secretion of paracrine factors that may stimulate the growth of latently infected B-cell lines [9, 11]. While it may have a more diffuse effect than the latent mechanism, EBV lytic infection mediated signalling may contribute to the development of lymphoproliferative disease. | ||
The lytic cycle begins with mature EBV virions reaching target host cells, such as B-cells. Contact between the virion and B-cell is initiated by the binding of EBV glycoprotein 350 to B-cell CD-21 [12]. | The lytic cycle begins with mature EBV virions reaching target host cells, such as B-cells. Contact between the virion and B-cell is initiated by the binding of EBV glycoprotein 350 to B-cell CD-21 [12]. EBV 350 is an example of a lytically expressed gene that is speifcially target by the human immune system [13]. This gp350 binding is complemented by the binding of EBV gp42 to B-cell MHC-II [14]. In order for fusion to occur between the viral envelope and B-cell membrane, the EBV virion must have functional gH, gL, and gp42 spike glycoproteins [15]. | ||
==Latent Life Cycle== | |||
==References== | ==References== | ||
| Line 44: | Line 46: | ||
[11] Hong, G. K., Gulley, M. L., Feng, W. H., Delecluse, H. J., Holley-Guthrie, E. and Kenney, S.C. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. Journal of Virology. 79: 13993–14003. | [11] Hong, G. K., Gulley, M. L., Feng, W. H., Delecluse, H. J., Holley-Guthrie, E. and Kenney, S.C. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. Journal of Virology. 79: 13993–14003. | ||
[12] Nemerow, G. R., Mold, C., Schwend, V. K., Tollefson, V. Cooper, N. R. 1987. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein–Barr virus(EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complment fragment C3d. J. Virol. 61: 1416–1420. | |||
[13] Khyatti M, Patel PC, Stefanescu I, Menezes J. 1991. Epstein-Barr virus (EBV) glycoprotein gp350 expressed on transfected cells resistant to natural killer cell activity serves as a target antigen for EBV-specific antibody-dependent cellular cytotoxicity. Journal of Virology. 65(2): 996-1001. | |||
[14] Borza, C. M. Hutt-Fletcher, L. M. 2002. Alternate replication in B-cells and epithelial cells switches tropism of Epstein–Barr | |||
virus. Nature Med. 8: 594–599. | |||
[15] Kirschner AN, Omerovic J, Popov B, Longnecker R, Jardetzky TS. 2006. Soluble Epstein-Barr Virus Glycoproteins gH, gL, and gp42 Form a 1:1:1 Stable Complex That Acts Like Soluble gp42 in B-Cell Fusion but Not in Epithelial Cell Fusion. Journal of Virology. 80(19): 9444-9454. | |||
Revision as of 04:36, 14 November 2012
Introduction
The Epstein-Barr Virus (EBV) is a common human herpes virus that can cause both infectious mononucleosis and lymphoproliferative disease. EBV is unique in that it infects about 95% of the adult population between 35-40 years old in the U.S. [1]. EBV is associated with cancers such as Burkitt’s Lymphoma and nasopharyngeal carcinoma [1,2]. The virus is capable of infecting host B-cells, and primarily proliferates via a non-lytic mechanism [2]. During this latent process, virus-derived nuclear proteins (EBNAs) and membrane proteins (LMPs) are expressed by infected host cells [2]. An advancing area of research is aimed at understanding how viral proteins may play a role in lymphoproliferative disease. LMP-1 is one of these viral membranous proteins that may induce indefinite, tumorigenic replication in infected B-cells [2,3]. In a sense, the LMP-1 acts to "transform" these B-cells into an immortalized, proliferating line. While EBV infections usually only cause mild symptoms, attempts to develop treatments and antivirals have generally been unsuccessful [1]. It is particularly difficult to control spread between hosts as chronic infections may reactivate, allowing symptom-free carriers to continue to transmit the virus years after initial infection [1]. Due to EBV's putative role in carcinogenesis and the limited success in development of antivirals to combat infection, it is essential to develop a deeper understanding of the mechanisms by which the virus alters the character of host cells.
Virus Classification and Genome
The Epstein-Barr virus is a Baltimore Class I virus of the family Herpesviridae. It is a gammaretrovirus also designated as Human Herpes Virus 4 (HHV-4). M.A. Epstein and Yvonne Barr were the first identify EBV in tumor tissue associated with Burkitt's Lymphoma. The researchers used electron microscopy to determine that these viral particles were very similar in structure to Herpes simplex virions [4]. The genome of EBV is composed of double-stranded DNA and is 172,282 base-pairs long [5]. This relatively long genome is characteristic of Herpes viruses; Herpes simplex has a 152-kilobase genome [6].
EBV Virion Structure
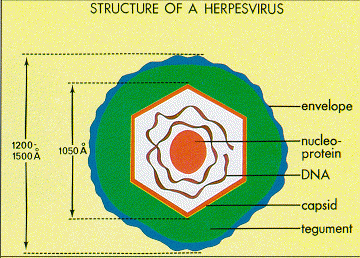
Epstein and Barr observed that this lymphoma-associated virus was about 20% smaller than typical Herpes simplex virions [4]. Similar to other Herpesviruses, the innermost part of the EBV virion consists of DNA wrapped around a central nucleo-protein core [7]. The core of the virion is surrounded by a nucleocapsid, a layer of protein tegument, and finally and outer envelope with spike glycoproteins [7]. Similar to many other viruses, many of these glycoproteins are vital for host-cell entry mechanisms. Infected host cells release EBV virions exclusively during the lytic cycle [8].
Lytic Life Cycle
While the EBV lytic life cycle is more rarely observed than the latent mode of replication, it is particularly important as it is the only way that the virus may make virions and be transferred horizontally between hosts (or cells) [8,9]. Immunosuppresive diseases like AIDS typically show increased free virion levels in the blood, a marker of increased lytic activity [9]. While virions are often found in the saliva of infected hosts, little to no lytic-infected cells are typically detected in the body [9]. Human cytotoxic T-cells are particurally adept at recognizing and destroying lytically infected cells expressing certain early stage lytic genes [10]. Hence, lytic activity appears to drive EBV spread in human populations. However, the latent replicative cycle is favored under normal conditions within a host, possibly as a means to evade host immune responses. Alpha and Beta hepesviruses have elaborate mechanisms for lytic gene concealment from the host immune system while EBV has few mechanisms to prevent immunomediated destruction of lytic-infected cells [10]. For example, Herpes simplex may be capable of inhibition of host Major Histocombatibility Complex, which reduces B-cell antigen presentation and recognition by cytotoxic T-cells [10]. In contrast to other herpesviruses, gammaherpesviruses like EBV are capable of proliferation via clonal replication within host B-cells [10]. Since EBV can replicate effectively via this latent system, there is likely a reduced selective pressure for elaborate immune-avoidance mechanisms in the lytic cycle. Said another way, in contrast to other herpes viruses, where lytic replication is essential for continued, proliferative infection in a host, lytic replication in EBV takes on a specific "niche role" as a mediator of host-host transmission.
While latent activity typically is the primary contributor to the development of lymphoproliferative diesease (LPD) in EBV infected individuals, lytic-incompetent EBV strains are less effective generators of LPD associated lesioning than the wild-type [11]. Lytic infection results in the secretion of paracrine factors that may stimulate the growth of latently infected B-cell lines [9, 11]. While it may have a more diffuse effect than the latent mechanism, EBV lytic infection mediated signalling may contribute to the development of lymphoproliferative disease.
The lytic cycle begins with mature EBV virions reaching target host cells, such as B-cells. Contact between the virion and B-cell is initiated by the binding of EBV glycoprotein 350 to B-cell CD-21 [12]. EBV 350 is an example of a lytically expressed gene that is speifcially target by the human immune system [13]. This gp350 binding is complemented by the binding of EBV gp42 to B-cell MHC-II [14]. In order for fusion to occur between the viral envelope and B-cell membrane, the EBV virion must have functional gH, gL, and gp42 spike glycoproteins [15].
Latent Life Cycle
References
[1]"Epstein-Barr Virus and Infectious Mononucleosis." Centers for Disease Control. http://www.cdc.gov/ncidod/diseases/ebv.htm. Accessed: 11/3/12.
[2]Young LS,Rickinson AB. 2004. Epstein-Barr Virus: 40 Years On. Nature Reviews. 4:757-768.
[3]Wang D, Liebowitz D, Kieff E. 1985. An EBV Membrane Protein Expressed in Immortalized Lymphocytes Transforms Established Rodent Cells. Cell. 43: 831-840.
[4]Epstein MA, Achong BG, Barr YM. 1964. Virus Particles In Cultured Lymphoblasts from Burkitt's Lymphoma. The Lancet. 283:702-703.
[5] Baer R, Bankier AT, Biggin MD, Deininger DL, Farrell PJ, Gibson TJ, Hatfull G, Hudson GS, Satchwell SC, Seguin C, Tuffnell PS, Barrell BG. 1984. DNA sequence and expression of the B95-8 Epstein-Barr Virus Genome. Nature. 310: 207-211.
[6] Mahiet C, Ergani A, Huot N, Alende N, Azough A, Salvaire F, Bensimon A, Conseiller E, Wain-Hobson S, Labetoulle M, Berradeau S. 2012. Structural Variability of the Herpes Simplex Virus 1 Genome in Vitro and In Vivo. Journal of Virology. 86(16): 8592-8601.
[7]Liebowitz D, Kieff E. 1993. Epstein-Barr virus. In: The Human Herpesvirus. Roizman B, Whitley RJ, Lopez C, editors. New York. 107–172.
[8] "Epstein-Barr Virus." International Agency for Research on Cancer. IARC Monographs. 1997. 49-92. http://monographs.iarc.fr/ENG/Monographs/vol100B/mono100B-6.pdf. Accessed: 11/3/12.
[9] Swaminathan S, Kenney S. "The Epstein-Barr Virus Lytic Life Cycle." 2009. In: DNA Tumor Viruses. 285-315.
[10] Steven, N. M., Annels, N. E., Kumar, A., Leese, A. M., Kurilla, M. G. and Rickinson, A. B. 1997. Immediate early and early lytic cycle proteins are frequent targets of the EpsteinBarr virus-induced cytotoxic T cell response. J Exp Med. 185: 1605–1617.
[11] Hong, G. K., Gulley, M. L., Feng, W. H., Delecluse, H. J., Holley-Guthrie, E. and Kenney, S.C. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. Journal of Virology. 79: 13993–14003.
[12] Nemerow, G. R., Mold, C., Schwend, V. K., Tollefson, V. Cooper, N. R. 1987. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein–Barr virus(EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complment fragment C3d. J. Virol. 61: 1416–1420.
[13] Khyatti M, Patel PC, Stefanescu I, Menezes J. 1991. Epstein-Barr virus (EBV) glycoprotein gp350 expressed on transfected cells resistant to natural killer cell activity serves as a target antigen for EBV-specific antibody-dependent cellular cytotoxicity. Journal of Virology. 65(2): 996-1001.
[14] Borza, C. M. Hutt-Fletcher, L. M. 2002. Alternate replication in B-cells and epithelial cells switches tropism of Epstein–Barr virus. Nature Med. 8: 594–599.
[15] Kirschner AN, Omerovic J, Popov B, Longnecker R, Jardetzky TS. 2006. Soluble Epstein-Barr Virus Glycoproteins gH, gL, and gp42 Form a 1:1:1 Stable Complex That Acts Like Soluble gp42 in B-Cell Fusion but Not in Epithelial Cell Fusion. Journal of Virology. 80(19): 9444-9454.
