Thermococcus fumicolans: Difference between revisions
mNo edit summary |
No edit summary |
||
| (11 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
| Line 26: | Line 23: | ||
==Description and Significance== | ==Description and Significance== | ||
''Thermococcus fumicolans'' is an archaeal [ | ''Thermococcus fumicolans'' is an [http://microbewiki.kenyon.edu/index.php/Taxonomy_Index archaeal] [http://en.wikipedia.org/wiki/Hyperthermophile hyperthermophile] found in deep-sea [[hydrothermal vent]] communities. It was isolated in 1997 by Godfroy et al. from vent chimney fragments taken from the North Fiji basin and classified in the genus ''Thermococcus'' based on its [[16S rRNA]] gene sequence. An [[obligate anaerobe]], it thrives in anoxic, sulfur-rich water being emitted from the vent at temperatures of 85 °C, with growth on amino acids and enhanced growth with elemental sulfur [1]. It is a [[chemoorganoheterotroph]] capable of surviving in harsher conditions than those characteristic of thermal vents, depending on nutrient and substrate availability [5]. Many ''T. fumicolans'' enzymes, such as ''Tfu'' DNA polymerase have very high thermostability and represent typical bacterial adaptations to the stresses of extreme environments. | ||
==Genome and Phylogeny== | ==Genome and Phylogeny== | ||
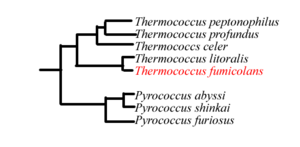
The genome of ''T. fumicolans'' has yet to be sequenced. Based on the 16S rRNA sequences, ''T. fumicolans'' is related to other ''Thermococcales'' species such as ''T. litoralis''. [1] The proliferating cell nuclear antigen (PCNA) of ''T. fumicolans'' is functionally conserved among hyperthermophiles, but is similar to that of mammals as well [7]. Members of the genus ''Thermococcus'' are sometimes found with large amounts of extracellular DNA in vesicles, thought to be of viral origin. These vesicles are effective in protecting DNA from thermal degradation and may be involved in horizontal gene transfer[12]. | The genome of ''T. fumicolans'' has yet to be sequenced. Based on the 16S rRNA sequences, ''T. fumicolans'' is related to other [[''Thermococcales'']] species such as ''T. litoralis''. [1] The [[proliferating cell nuclear antigen]] (PCNA) of ''T. fumicolans'' is functionally conserved among hyperthermophiles, but is similar to that of mammals as well [7]. Members of the genus ''Thermococcus'' are sometimes found with large amounts of extracellular DNA in vesicles, thought to be of viral origin. These vesicles are effective in protecting DNA from thermal degradation and may be involved in [[horizontal gene transfer]][12]. | ||
[[Image:Phylogeny_tfu.png|thumb|300px| Phylogenetic tree showing the genus Thermococcus. Adapted from source [1].]] | |||
==Cell Structure, Metabolism and Growth== | ==Cell Structure, Metabolism and Growth== | ||
[[ | ''T. fumicolans'' is a [[Gram-negative]] [[coccus]] about 0.8 to 2 pm in diameter [1]. The cells are motile, possessing polar [[flagella]], and grow in singles, pairs, or small clusters. | ||
The cell envelope consists of two layers and contains glycerol ether lipids, 70% of which are diphytanyl glycerol ether, characteristic of Archaea. [1]. | |||
''T. fumicolans'' is | ''T. fumicolans'' is an obligate [[chemoorganoheterotroph]] and an anaerobe [3]. It grows on [[proteolysis]] products and various amino acids. Sulfur increases growth but is not required [1]. In the absence of other nutrients, ''T. fumicolans'' are characteristically adapted to grow on pyruvate alone. This ability has not been demonstrated in ''Thermococcus'' species other than ''T. fumicolans'' and ''T. litoralis'' [8] [1]. | ||
''T. fumicolans'' grows at a temperature range of 73-100°C with the optimum being 85-90 °C, the highest of all known ''Thermococcus'' species [1]. Although it grows at a pH range of 4.5 to 9.5, the optimal pH is approximately 8. Beyond 100°C, ''T. fumicolans'' is no longer thermotolerant and cannot survive at any pH [5]. During growth phases, ''T. fumicolans'' can tolerate extreme acidity, pressure and temperature exceeding those of most hydrothermal vent environments. However, when substrates are lacking, survival is drastically decreased except under milder conditions [8]. ''T. fumicolans'' and hyperthermophiles such as [[''M. jannaschii'']] and [[''A. profundus'']] need energy and carbon sources even in non-growth stages, and are unlikely to become [[metabolically inactive]]. These [[extremophiles]] undergo active biosynthesis in order to survive in high-stress environments [5]. It is suggested that ''T. fumicolans'' divides by binary fission along the middle of the cell [1], and has a doubling time of less than 86 minutes [6]. | |||
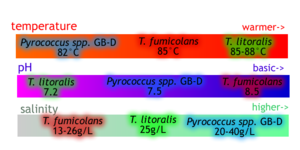
[[Image:Thermococcus_compare.png|thumb|300px|left| Diagram comparing optimal growth conditions for T. fumicolans and several related species. Data from sources [8] and [1]]] | |||
''T. fumicolans'' shows resistance to vancomycin, penicillin, kanamycin, streptomycin, and chloramphenicol, but is not resistant to rifampin at the same concentrations [1]. | ''T. fumicolans'' shows resistance to [[vancomycin]], [[penicillin]], [[kanamycin]], [[streptomycin]], and [[chloramphenicol]], but is not resistant to [[rifampin]] at the same concentrations [1]. | ||
==Ecology== | ==Ecology== | ||
''Thermococcus fumicolans'' is found in hydrothermal environments with anhydrite chimneys, and grows alongside ''Pyrococcus spp''. GB-D [5] and various methanogens, sulfate reducers and sulfur metabolizers [1]. ''Thermococcales'' have been shown to carry viral particles, which are important in controlling microbial populations in the ocean [12]. | ''Thermococcus fumicolans'' is found in [[hydrothermal environments]] with anhydrite chimneys, and grows alongside [[''Pyrococcus spp''.]] GB-D [5] and various [[methanogens]], [[sulfate reducers]] and sulfur metabolizers [1]. ''Thermococcales'' have been shown to carry viral particles, which are important in controlling microbial populations in the ocean [12]. | ||
==Enzyme structure and activity== | ==Enzyme structure and activity== | ||
The two intein genes, ''Pol-1'' and ''Pol-2'', of ''T. fumicolans'' DNA polymerase were shown to have endonuclease activity after recombination into the ''Escherichia coli'' genome [6]. ''T. fumicolans'' possesses amylolytic enzymes that could be capable of degrading glycogetis from animal biomass. Fallen biomass and other detritus are readily available in hydrothermal vents, which generally lack vegetation [9]. The ''Tfu'' DNA polymerase found in ''T. fumicolans'' is more thermostable than ''Tli'' DNA polymerase and ''Taq'' polymerase, and can polymerize mammalian DNA at high temperatures. ''Tfu'' polymerase activity decreases at high temperatures but it is suggested that this is due to denaturing of substrate rather than polymerase instability [6]. | The two [[intein]] genes, ''Pol-1'' and ''Pol-2'', of ''T. fumicolans'' [[DNA polymerase]] were shown to have [[endonuclease] activity after [[recombination]] into the ''[[Escherichia coli]]'' genome [6]. ''T. fumicolans'' possesses amylolytic enzymes that could be capable of degrading glycogetis from animal biomass. Fallen biomass and other detritus are readily available in hydrothermal vents, which generally lack vegetation [9]. The ''Tfu'' DNA polymerase found in ''T. fumicolans'' is more thermostable than ''Tli'' DNA polymerase and ''Taq'' polymerase, and can polymerize mammalian DNA at high temperatures. ''Tfu'' polymerase activity decreases at high temperatures but it is suggested that this is due to denaturing of substrate rather than polymerase instability [6]. | ||
==Biochemical applications== | ==Biochemical applications== | ||
| Line 84: | Line 84: | ||
12. Soler, N., Marguet, E., Verbavatz, J., and Forterre, P. 2008. Virus-like vesicles and extracellular DNA produced by hyperthermophilic archaea of the order Thermococcales. Res. In Microbiol. 159: 390-399 | 12. Soler, N., Marguet, E., Verbavatz, J., and Forterre, P. 2008. Virus-like vesicles and extracellular DNA produced by hyperthermophilic archaea of the order Thermococcales. Res. In Microbiol. 159: 390-399 | ||
Latest revision as of 21:45, 1 December 2015
Thermococcus fumicolans
Classification
Higher order taxa:
Archaea; Euryarchaeota; Thermococci; Thermococcales; Thermococcaceae
Species:
Thermococcus fumicolans [1]
|
NCBI: [1] |
Description and Significance
Thermococcus fumicolans is an archaeal hyperthermophile found in deep-sea hydrothermal vent communities. It was isolated in 1997 by Godfroy et al. from vent chimney fragments taken from the North Fiji basin and classified in the genus Thermococcus based on its 16S rRNA gene sequence. An obligate anaerobe, it thrives in anoxic, sulfur-rich water being emitted from the vent at temperatures of 85 °C, with growth on amino acids and enhanced growth with elemental sulfur [1]. It is a chemoorganoheterotroph capable of surviving in harsher conditions than those characteristic of thermal vents, depending on nutrient and substrate availability [5]. Many T. fumicolans enzymes, such as Tfu DNA polymerase have very high thermostability and represent typical bacterial adaptations to the stresses of extreme environments.
Genome and Phylogeny
The genome of T. fumicolans has yet to be sequenced. Based on the 16S rRNA sequences, T. fumicolans is related to other ''Thermococcales'' species such as T. litoralis. [1] The proliferating cell nuclear antigen (PCNA) of T. fumicolans is functionally conserved among hyperthermophiles, but is similar to that of mammals as well [7]. Members of the genus Thermococcus are sometimes found with large amounts of extracellular DNA in vesicles, thought to be of viral origin. These vesicles are effective in protecting DNA from thermal degradation and may be involved in horizontal gene transfer[12].
Cell Structure, Metabolism and Growth
T. fumicolans is a Gram-negative coccus about 0.8 to 2 pm in diameter [1]. The cells are motile, possessing polar flagella, and grow in singles, pairs, or small clusters.
The cell envelope consists of two layers and contains glycerol ether lipids, 70% of which are diphytanyl glycerol ether, characteristic of Archaea. [1].
T. fumicolans is an obligate chemoorganoheterotroph and an anaerobe [3]. It grows on proteolysis products and various amino acids. Sulfur increases growth but is not required [1]. In the absence of other nutrients, T. fumicolans are characteristically adapted to grow on pyruvate alone. This ability has not been demonstrated in Thermococcus species other than T. fumicolans and T. litoralis [8] [1].
T. fumicolans grows at a temperature range of 73-100°C with the optimum being 85-90 °C, the highest of all known Thermococcus species [1]. Although it grows at a pH range of 4.5 to 9.5, the optimal pH is approximately 8. Beyond 100°C, T. fumicolans is no longer thermotolerant and cannot survive at any pH [5]. During growth phases, T. fumicolans can tolerate extreme acidity, pressure and temperature exceeding those of most hydrothermal vent environments. However, when substrates are lacking, survival is drastically decreased except under milder conditions [8]. T. fumicolans and hyperthermophiles such as ''M. jannaschii'' and ''A. profundus'' need energy and carbon sources even in non-growth stages, and are unlikely to become metabolically inactive. These extremophiles undergo active biosynthesis in order to survive in high-stress environments [5]. It is suggested that T. fumicolans divides by binary fission along the middle of the cell [1], and has a doubling time of less than 86 minutes [6].
T. fumicolans shows resistance to vancomycin, penicillin, kanamycin, streptomycin, and chloramphenicol, but is not resistant to rifampin at the same concentrations [1].
Ecology
Thermococcus fumicolans is found in hydrothermal environments with anhydrite chimneys, and grows alongside ''Pyrococcus spp''. GB-D [5] and various methanogens, sulfate reducers and sulfur metabolizers [1]. Thermococcales have been shown to carry viral particles, which are important in controlling microbial populations in the ocean [12].
Enzyme structure and activity
The two intein genes, Pol-1 and Pol-2, of T. fumicolans DNA polymerase were shown to have [[endonuclease] activity after recombination into the Escherichia coli genome [6]. T. fumicolans possesses amylolytic enzymes that could be capable of degrading glycogetis from animal biomass. Fallen biomass and other detritus are readily available in hydrothermal vents, which generally lack vegetation [9]. The Tfu DNA polymerase found in T. fumicolans is more thermostable than Tli DNA polymerase and Taq polymerase, and can polymerize mammalian DNA at high temperatures. Tfu polymerase activity decreases at high temperatures but it is suggested that this is due to denaturing of substrate rather than polymerase instability [6].
Biochemical applications
Hydrothermal vent environments may be an important source for new thermostable enzymes [9]. Because Tfu DNA polymerase maintains its structure in extremely high heat, it could be practical in various molecular biology applications, similar to the use of Taq DNA polymerase in the polymerase chain reaction which occurs at 94°C [4]. Some of the proteins unique to Thermococcales could be useful for stabilizing other compounds such as lipids for use in reactions at high temperatures[11].
References
1. Godfroy, A., Meunier, J., Guezennec, J., Lesongeur, F., Raguenes, G., Rimbault, A., and Barbier, G. 1996. Thermococcus fumicolans sp. nov., a New Hyperthermophilic Archaeon Isolated from a Deep-Sea Hydrothermal Vent in the North Fiji Basin. Int. J. Systematic Bacteriology 46: 1113-1119
2. Thermococcus fumicolans genome sequence. http://www.ncbi.nlm.nih.gov/
3. Cambon-Bonavita, M., Schmitt, P., Zieger, M., Flaman, J., Lesongeur, F., Raguenes, G., Bindel, D., Frisch, N., Lakkis, Z., Dupret, D., Barbier, G., and Querellou, J. 2000. Cloning, expression and characterization of DNA polymerase I from the hyperthermophilic archaea Thermococcus fumicolans. Extremophiles 4: 215-225
4. Raffin, J., Henneke, G., and Dietrich, J. 2000. Purification and characterization of a new DNA polymerase modulator from the hyperthermophilic archaeon Thermococcus fumicolans. Comp. Bioc. & Physiol. Part B 127: 299-308
5. Lloyd, K. G., Edgcomb, V. P., Molyneaux, S. J., Boer, S., Wirsen, C. O., Atkins, M. S., and Teske, A. 2005. Effects of Dissolved Sulfide, pH and Temperature on Growth and Survival of Marine Hyperthermophilic Archaea. Appl. Environ. Microbiol. 71(10): 6383-6387
6. Saves, I., Ozannes, V., Dietrich, J., and Masson, J. 2000. Inteins of Thermococcus fumicolans DNA Polymerase Are Endonucleases with Distinct Enzymatic Behaviors. J. Biol. Chem. 275(4): 2335-2341
7. Henneke, G., Raffin, J., Ferrari, E., Jonsson, Z. O., Dietrich, J., and Hubscher, U. 2000. The PCNA from Thermococcus fumicolans Functionally Interacts with DNA Polymerase d. Bioc. & Biophys. Research Comm. 276: 600-606
8. Edgcomb, V. P., Molyneaux, S. J., Boer, S., Wirsen, C. O., Saito, M., Atkins, M. S., Lloyd, K., and Teske, A. 2007. Survival and growth of two heterotrophic hydrothermal vent archaea, Pyrococcus strain GB-D and Thermococcus fumicolans under low pH and high sulfide concentrations in combination with high temperature and pressure regines. Extremophiles 11: 329-342
9. Legin, E., Ladrat, C., Godfroy, A., Barbier, G., and Duchiron, F., 1997. Thermostable amylolytic enzymes of thermophilic microorganisms from deep-sea hydrothermal vents. C. R. Acad. Sci. Paris. Sciences de la vie/Life Sciences 320: 893-898
10. Edgcomb, V. P., Molyneaux, S. J., Boer, S., Wirsen, C. O., Saito, M., Atkins, M. S., Lloyd, K., and Teske, A. 2004. Sulfide Ameliorates Metal Toxicity for Deep-Sea Hydrothermal Vent Archaea. Applied and Environmental Microbiol. 70(4): 2551-2555
11. Turner, P., Mamo, G., and Karlsson, E.N. 2007. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 6(9)
12. Soler, N., Marguet, E., Verbavatz, J., and Forterre, P. 2008. Virus-like vesicles and extracellular DNA produced by hyperthermophilic archaea of the order Thermococcales. Res. In Microbiol. 159: 390-399