Thiobacillus denitrificans
Classification
Species
Description and Significance
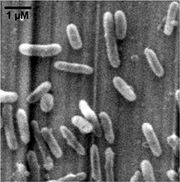
Thiobacillus denitrificans is short rod-shaped (0.5×1.0-3.0 um), Gram-negative, obligately chemolithoautotrophic, and a member of the beta- subclass of the Proteobacteria. Different from many known chemolithotrophic sulfur-oxidizing bacteria (such as Acidithiobacillus ferrooxidans) which are strictly aerobic, T. denitrificans grows as a facultatively anaerobic chemolithotroph, coupling the oxidation of inorganic sulfur compounds to the reduction of nitrate, nitrite and other oxidized nitrogen compounds to dinitrogen. The optimum conditions for denitrification are pH 6.85 at 32.8°C, while the optimal growth conditions are pH 6.90 at 29.5°C. Found in soil, mud, freshwater- and marine sediments and also in domestic sewage and industrial waste-treatment lagoons and digestion tanks, especially under anoxic conditions.
T. denitrificans can remediate natural groundwater and engineered water treatment systems by removing excess nitrate. It is the first and only autotrophic bacterium reported to carry out anaerobic (nitrate-dependent) oxidation of U(IV) oxide minerals, which could partially counteract efforts to remediate uranium contaminated aquifers by in situ reductive immobilization
T. denitrificans is one of the best studied of the very few obligately chemolithoautotrophic species which can couple denitrification with sulfur-compound oxidation.
Genome Structure

Cell Structure, Metabolism and Life Cycle
The cells may be motile through a polar flagellum. Clear or weakly opalescent colonies are grown anaerobiclally on thiosulfate/nitrate agar, which upon aging may become white with sulfur. Growth in anaerobic stab- or roll culture results in agar splitting due to production of nitrogen gas.
T. denitrificans grows slowly with no apparent stable phase. The maximal denitrification rate reached 2.245 mg/(L-h), which is found in exponential phase. In the process of the culture, the medium pH decreases significantly. Relatively high salinity restrains the denitrification activity of T. denitrificans. The acute toxicity test results show that T. denitrificans is not toxic.
T. denitrificans grows aerobically on thiosulfate, tetrathionate and thiocyanate; it also grows anaerobically on thiosulfate, tetrathionate, thiocyanate, sulfide or elemental sulfur.
2NO3- + S + H2O + CaCO3 --> CaSO4 + N2
Previous chemical equation represents one of the typical reactions coupling the oxidation of inorganic sulfur compounds to the reduction of oxidized nitrogen compounds. The inorganic sulfur compounds provide electrons, while the oxidized nitrogen compounds behave has terminal respiratory oxidants. Ammonium salts and, in some strains at least, nitrate are used as nitrogen sources. There is evidence that in T. denitrificans, sulfide is oxidized by a series of reactions catalyzed by a sulfide/sulfite oxidoreductase similar to that found in some sulfate-reducing bacteria. The oxidation of elemental sulfur by T. denitrificans might be preceded by its initial reduction to sulfide, followed by oxidation to sulfite.
In addition, most electron donors that T. denitrificans can utilize are poorly-soluble minerals containing reduced iron and/or sulfur, such as pyrite (FeS2) and FeS. The mechanism by which this species can use solid-phase electron donors that cannot be taken into the cell is of considerable interest but is currently unknown.
Studies on fixation of carbon dioxide of T. denitrificans found that it is capable of synthesizing hexose phosphates from carbon dioxide by a cyclic mechanism similar to that found in green plants.
Batch cultures can be grown in completely filled bottles, producing vigorous nitrogen evolution. Chemostat culture can be switched easily and repeatedly between aerobic and anaerobic growth modes, with adaptation involving derepression of nitrate and nitrite reductase synthesis.
Ecology and Pathogenesis
References
- Kelly, D.P., A.P. Wood, 2000. Confirmation of Thiobacillus denitrificans as a species of the genus Thiobacillus, in the beta-subclass of the Proteobacteria, with strain NCIMB 9548 as the type strain. International Journal of Systematic and Evolutionary Microbiology, 50: 547-440
- Shao, M., T. Zhang, H.H. Fang, 2010. Sulfur-driven autotrophic denitrification: diversity, biochemistry, and engineering applications. Applied Microbiology and Biotechnology, 88 (5): 1027-1042
- Karyn’s Genomes, European Bioinfomatics Institute.
- Lengeler, J.W., G. Drews, H.G. Schlegel, 1999. Oxidation of Inorganic Compounds by Chemolithotrophs. Biology of the prokaryotes, 10: 234-260. [ISBN 3-13-108411-1]
Authors
Page authored by Emily Campbell and Rui Chen, student of Prof. Jay Lennon at Michigan State University.
