User:Rachelso2020/Dallol: Difference between revisions
Rachelso2020 (talk | contribs) No edit summary |
Rachelso2020 (talk | contribs) No edit summary |
||
| Line 81: | Line 81: | ||
[[Image: Arabia Terra on Mars.JPG|thumb|700px|center| Satellite images spring mounds (left) and layered deposits (right) in Arabia Terra on Mars.<ref name= Cavalazzi/>]] | [[Image: Arabia Terra on Mars.JPG|thumb|700px|center| Satellite images spring mounds (left) and layered deposits (right) in Arabia Terra on Mars.<ref name= Cavalazzi/>]] | ||
==Conclusion== | ==Conclusion== | ||
Revision as of 19:47, 6 June 2020
Dallol Hydrothermal System
Overview

By Rachel So
Date updated: June 3, 2020
The Dallol hydrothermal system is in the northern part of the Danakil Depression in the Afar Triangle (also called Afar triple junction or Afar Depression) of northeastern Ethiopia.[1][2] Dallol is a desert that receives less than 200 mm of rainfall a year and holds the record as the hottest location on the planet (mean annual temperature of 34.5°C).[3][4] Its hydrothermal pools form a unique hyperthermal (25-110°C), hypersaline (33% to > 50% salinity), and hyperacidic (pH < -1.5 to 6.0) polyextreme environment that pushes the limits of life.[5] In addition, the pools contain high concentrations of chlorides, sulfates, and dissolved iron (both Fe2+ and Fe3+), the last giving them their colorful appearance.[1] Once thought to be absent of any lifeforms due to the harsh conditions, recent research have discovered a wide variety of microbes living in different locations within the Dallol hydrothermal system.[2][5][6] These include several phyla of archaea and bacteria, and both extremophiles and not.
About the Environment
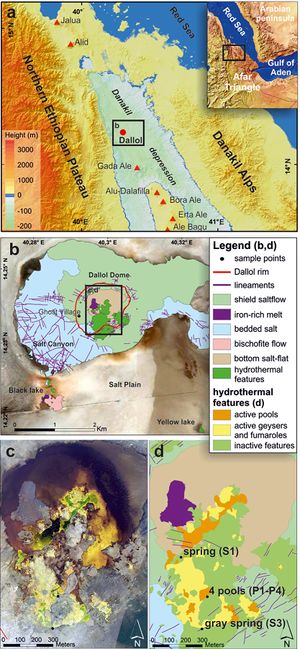
Located at the junction of the Nubian, Somali, and Arabian plates, the Danakil Depression is part of the East African Rift system and experiences high tectonic and geothermal activity.[3][7] The Depression exists at the northern end of a line of Holocene-age active volcanoes that run north-northwest to south-southeast.[3] While not a proper volcano itself, its physical appearance, geothermal activity, and location along this volcanic range has caused the Dallol dome (within the Depression) to sometimes be referred to as the "Dallol volcano" or "Dallol protovolcano".[1][3] Despite its occasional designation as a volcano, the Dallol dome does not experience eruptions or have volcanic outcrops.[5]

The Danakil Depression is approximately 120 m below sea level with the Dallol dome rising up about 40 m from the Depression. [5] The area around the dome is a vast salt plain composed of 2 km thick evaporites (enriched in potassium, manganese, iron, magnesium, and zinc) deposited by repeated transgressions of the Red Sea.[1][8] The continental crust at Danakil is less than 15 km thick, a result of variable rifting over much of the Afar region.[7]
A large variety of hydro-geothermal features are found at Dallol including hydrothermal springs, brine pools, geysers, and salt chimneys, pillars, and terraces.[1] These features are formed from evaporation of supersaturated brines and are highly dynamic.[1] The number of active springs changes year to year and some pools appear and disappear over periods of days to weeks.[1][7] A number of unique precipitate formations have also been recorded including, but not limited to, flower-like structures, eggshell-like structures, spherules, and thick crusts cracked into polygons.[1]
Due to the area's heterogeneity, water samples collected from Dallol vary in temperature, pH, salinity, and solute composition and concentrations. Temperatures over 50°C, pH < 0, and salinities over 30% are common.[1][5][7] Several locations within the Dallol hydrothermal system have been studied including the hot springs and pools of the Dallol dome, Gaet'ale Lake (also called Yellow Lake) 4 km southeast of the dome, and Black Lake less than 2.5 km southwest of the dome.[1][2][5][7]
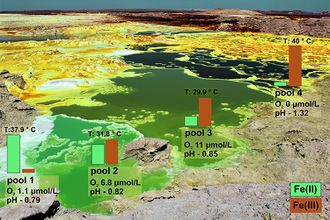
At the dome hot springs, the anoxic, hyperacidic, iron-rich source water from vents had recorded temperatures of 105-108°C.[1] As the water emerges, the temperature quickly decreases causing supersaturation and rapid halite precipitation. The water flows into pools, moving progressively further away from the spring, falling to temperatures of around 30°C. These waters have pH values of 0 or lower and salinities ranging from 37%-42%.[1][5]
A well-known feature of the Dallol pools is their bright colors including shades of greens, blues, yellows, and browns.These colors have been attributed mainly to iron redox chemistry. High concentrations of dissolved iron (22.5 g/L), sulfates (~5200 ppm), and chlorides (>200 g/L) contribute to the formation of colorful iron hydroxo-complexes, iron chloro-complexes, and iron sulfates. Spring water start out with high concentrations of ferrous iron (Fe2+) that is slowly oxidized (as the water equilibrates with the atmosphere) to ferric iron (Fe3+), causing pool waters to move from bright green to dark green to brown. Outside the water, thin layers of precipitated iron-(oxy)hydroxides and iron sulfates over the halite structures give them a similarly colorful appearance.[1]
While the age of the Dallol Dome is not known, Black Lake and Gaet'ale Lake appeared after phreatic explosions in 1926 and 2005, respectively.[1] For Black Lake, Cavalazzi et al. recorded an average temperature of 56°C and a pH of 1.4.[7] Belilla et al. found slightly different conditions at 70°C and pH 3 along with salinities >70%.[5] For Gaet'ale Lake, a temperature of 55°C and a pH of 3 were measured by the former, while 40°C and pH 1.8 were recorded by the latter (as well as salinities ≥ 50%).[7][5] Both lakes contain high concentrations of calcium (Ca2+) and magnesium (Mg2+) (particularly magnesium chloride), and Gaet'ale Lake also continuously bubbles up toxic gas.[5]
Dallol Microbial Ecology
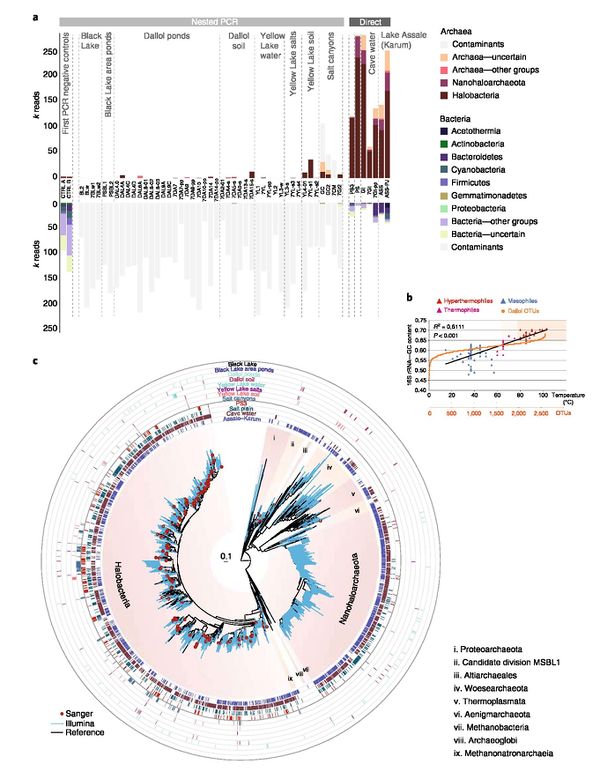
Much research has been conducted over the past two decades on the geology (e.g. formations, volcanic activity, seismicity) of the Dallol region.[9][10][11] However, studies on the diversity of life at Dallol have only been published in the last few years. Until recently, it was believed that Dallol hosted conditions too harsh for life.[1] New research using next generation sequencing (new and recent technology allowing for rapid, high through-put genome, transcriptome, etc. sequencing) and biomarker analysis suggests that microbial life is indeed present though their spatial distribution and general abundance is relatively low.[5]
Belilla et al. identified diverse archaeal and some bacterial taxa at certain sites in the Dallol geothermal system.[5] Water and geological samples were collected from the Dallol dome pools, nearby salt plains, Black Lake, Lake Gaet'ale, and Lake Assale (also Lake Karum; 30 km south of the dome). Sequencing and culturing only found evidence of microbes in the salt plain samples (~30°C, pH ~4-6, ~35% salinity) and in Lake Assale (~24°C, pH ~6.5, 36% salinity), both notably having milder conditions than other areas in Dallol.[5] The majority of sequences identified were archaeal, most of them halophiles with some thermophiles, though bacterial sequences were also found (mostly halophiles). Though not abundant, several archaeal taxa not typically associated with hypersaline environments were also identified including Thermoplasmata, Archaeoglobi, Thaumarchaeota, Crenarchaeota, amongst others. Under optical analysis, many of the cells were found to be very small.
Another study looking at precipitate samples found lipid biomarkers associated with select bacterial taxa including Aquificales, Thermotogales, and Chloroflexi.[6] In terms of metabolisms, Carrizo et al. suggested the presence of pristane and phytane, in the absence of any vegetation, could be due to microbial phototrophy. They also found enriched sulfate δ34S and enriched total sulfur δ34S, which they attribute to bacterial sulfate reduction. Overall, lipid biomarker evidence from Dallol precipitates suggests recent bacterial metabolism (or very well preserved biosignatures).
In addition to microbial life indicated from gene sequencing and biomarkers, electron microscopy also uncovered several abiotic biomorphs in Dallol water samples.[5] Biomorphs are mineral structures that resemble cells, but are not actually alive nor made by organisms.[5] The ones discovered at Dallol were primarily composed of silica though biomorphs composed of or rich in magnesium, calcium, and sulfur were also detected. Silica-encrustment of cells were also observed alongside biomorphs. Because biomorphs occur in Dallol, Belilla et al. recommended that future studies should incorporate a variety of techniques before claiming the presence of life.[5]
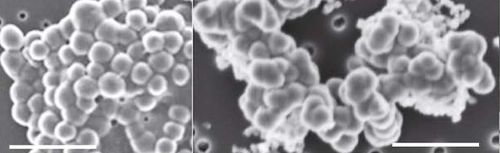
Key Microbial Players

Halobacteria/Haloarchaea
Halobacteria/Haloarchaea is an archaeal class in the phylum Euryarchaeota (note that there is also a genus called Halobacterium within this class).[12] It is sometimes referred to as "Halobacteria" despite being archaea because the genus Halobacterium was discovered prior to the discovery of the archaeal domain.[13] It is composed of the orders Halobacteriales, Natrialbales, and Haloferacales.[12][13] Members of this class are halophiles and most of them have growth optima at salinities of 12-23%.[13] Halobacteria are well-studied, many of its genera having been cultured, and its phylogenetic tree is also well-characterized.[12][13] Haloarchaea are gram-negative, do not have resting stages or spores, and have observed shapes of rods, cocci, cups, and more (e.g. Haloquadratum's square-shaped cells).[13] Most are oligate aerobes and many produce gas vesicles that allow them to float in water and be exposed to the air.[13] Halobacteria are chemoorganoheterotrophs, but some species can utilize light for ATP synthesis.[13] These species have red-colored C50 carotenoids as their main pigment and their form of phototrophy does not fix carbon dioxide.[13][14]
Nanohaloarchaea
Nanohaloarchaea is an archael class in the phylum Euryarchaeota. [15] Members of Nanohaloarchaea are ultrasmall halophiles (<0.8 μm in diameter) with reduced genomes (~1.2 Mb). [15] They have unusually low G+C content for extreme halophilic archaea and lack genes for making gas vesicles (see Halobacteria/Haloarchaea).[15] It has been hypothesized that the their small cell sizes allow them to float in water without the need for gas vesicles.[15] Similar to Halobacteria, Nanohaloarchaea are primarily aerobic chemoorganoheterotrophs, but have a rhodopsin-like gene that may allow for facultative photoheterotrophy.[16] So far, no Nanohaloarchaeal member has been successfully cultivated.[17] In addition, its position on the Archael phylogenetic tree is still being debated. While some studies have grouped Nanohaloarchaea along with other relatively new ultrasmall archaeal lineages in the DPANN superphylum, other studies suggest that Halobacteria and Nanohaloarchaea are sister lineages.[17] A recent study found evidence in support of the latter, suggesting that both lineages actually descended from two distinct branches of the methanogen Class II lineage.[17] This implies that two of the main halophilic archaeal lineages evolved independently from two methanogen ancestors.[17]
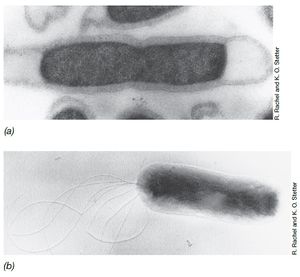
Bacterial Taxa
Several bacterial taxa were identified at Dallol using biomarker analysis including Aquificales, Thermotogales, and Chloroflexi.[6] Though present, research so far indicates bacterial taxa are less abundant than archaeal taxa and the aforementioned bacterial taxa were only found in geological samples.[5][6] Aquificales is a thermophilic and hyperthermophilic bacterial order that has been cultured.[18] Most are obligate or facultative chemolithoautotrophs using a variety of electron donors including hydrogen, sulfur, sulfate, thiosulfate, iron, and more.[18] Aquificales is commonly placed close to Thermotogales on the phylogenetic tree though it has also been proposed that Aquificales is more related to Proteobacteria.[18]
Similar to Aquificales, Thermotogales is an order known for its thermophiles and hyperthermophiles (though new studies have discovered low temperature species in Thermotogales).[19] Several thermophilic species inThermotogales have been cultured.[19] Thermotogales members mainly grow anaerobically as chemoorganoheterotrophs that ferment sugar.[13][19] Extensive horizontal gene transfer between Firmicutes and Thermotogales as well as between archaea and Thermotogales (which may contribute to the latter's thermophilic nature) have made determing Thermotogales's position on the phylogenetic tree difficult though some studies suggest Thermotogales to be a sister lineage to Aquificales.[20]
The class Chloroflexi contains green nonsulfur bacteria, which are mixotrophs that can grow as photoorganoheterotrophs (using simple organic carbon compounds as electron donors) or photolithoautotrophs (using hydrogen or hydrogen sulfide as donors).[13] Many species can also grow in the dark using aerobic respiration.[13] Several Chloroflexi representatives have been cultured. In Dallol, it's possible that Chloroflexi species are growing photolithoautotrophically using the abundant sulfur precipitates as electron donors. Several genera are also known thermophiles meaning high temperatures at Dallol (though not extremely high temperatures) might not be a problem.
Testing the Limits of Life
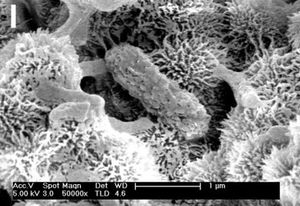
Dallol is an environment that combines temperature, pH, and salinity extremes into a single polyextreme environment, testing where life can exist and where it cannot. The heterogeneity of Dallol offers several sites with varying degrees of each extreme (e.g. Lake Assale vs. Black Lake) for inter-site comparisons.[5] Numerous microbe species living in extreme environments (e.g. thermophiles in high temperatures, acidophiles in low pH, halophiles in high salinity) have been studied[7] as well as some polyextremophiles such as those from thermo-acidic environments[21] and thermo-alkaline environments [22]. Until the recent studies on Dallol, microbes have never been identified in a high temperature, acidic, and high salinity environment.[7] The presence of microbes in Lake Assale and the salt plains, and their absence in the Dallol dome ponds, Black Lake, and Gaet'ale Lake provided some clues about the limits of life.[5] From these samples, two barriers to life have been hypothesized: 1) high chaotropicity and low water activity (due to high Mg2+ concentrations at Black Lake and Gaet'ale Lake), and 2) the co-occurrence of hyperacidity and hypersalinity (found at Dallol dome ponds).[5] In addition, the microbial cells detected using optical techniques are ultrasmall, between 0.1 and 0.2 μm, not easy to isolate using filters not easy to visualize.[2][5]
Analog for Extraterrestrial Environments
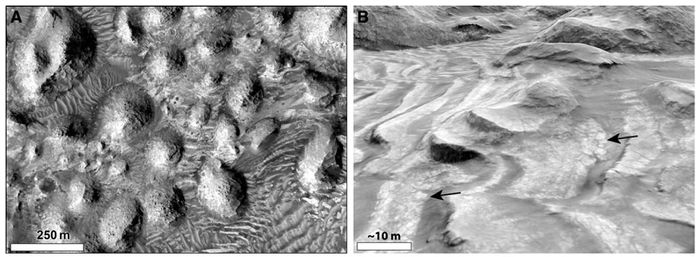
The Dallol hydrothermal system has been proposed as an analogue for extraterrestrial environments that could sustain life including Mars, Jupiter's moon Europa, and Saturn's moon Enceladus.[7][6] In general, hydrothermal systems have been proposed as sites for the origin of early life.[7] Hydrothermalism has been detected in extraterrestrial environments like Mars, making them promising locations for the detection of life beyond Earth.[7] Mars in particular hosts several sites very similar to Dallol, particularly in Mars's vast evaporite deposits and fluid dynamics (which occurred at some point in Mars's geological history).[7] For instance, Arabia Terra is a desert region on Mars with spring deposits and features indicating fluid expulsion.[7] It also contains extensive salt flats with evidence of dissolution and the presence of sulfate, making Arabia Terra similar to Dallol's salt plains.[7] Beyond Dallol's hydrothermal activity, its aquatic environments offer different clues for the study of extraterrestrial life. The presence of liquid water is considered a basic requirement for life, but the Dallol pools are proof that some waters are simply uninhabitable.[5] This places more limits towards what astronomers can consider extraterrestrial sites with the potential for life.
Conclusion
Extensive research has been conducted on the Dallol hydrothermal system's geology including its geochemistry and volcanic activity. Only recently have we begun to understand the distribution and diversity of Dallol's microbial community, which we formerly thought to be nonexistent. The detection of life is only the beginning. Dallol's heterogeneity means many locations within the region have yet to be studied. Future research can expand the known microbial diversity at Dallol as well as uncover life in new locations. Such efforts may even contribute to our knowledge of where life can exist beyond Earth.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 Kotopoulou, E., Huertas, A. D., Garcia-Ruiz, J. M., Dominguez-Vera, J. M., Lopez-Garcia, J. M., Guerra-Tschuschke, I., & Rull, F. (2019). A Polyextreme Hydrothermal System Controlled by Iron: The Case of Dallol at the Afar Triangle. Acs Earth and Space Chemistry, 3(1), 90-99. Article.
- ↑ 2.0 2.1 2.2 2.3 2.4 Gomez, F., Cavalazzi, B., Rodriguez, N., Amils, R., Ori, G. G., Olsson-Francis, K., et al. (2019). Ultra-small microorganisms in the polyextreme conditions of the Dallol volcano, Northern Afar, Ethiopia. Scientific Reports, 9, 9. Article.
- ↑ 3.0 3.1 3.2 3.3 Lopez-Garcia, J. M., Moreira, D., Benzerara, K., Grunewald, O., & Lopez-Garcia, P. (2020). Origin and Evolution of the Halo-Volcanic Complex of Dallol: Proto-Volcanism in Northern Afar (Ethiopia). Frontiers in Earth Science, 7, 24. Article.
- ↑ Pedgley, D. E. (1967). Air temperature at Dallol Ethiopia. Meteorological Magazine, 96(1142), 265-&. Article.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 Belilla, J., Moreira, D., Jardillier, L., Reboul, G., Benzerara, K., Lopez-Garcia, J. M., et al. (2019). Hyperdiverse archaea near life limits at the polyextreme geothermal Dallol area. Nature Ecology & Evolution, 3(11), 1552-+. Article.
- ↑ 6.0 6.1 6.2 6.3 6.4 Carrizo, D., Sanchez-Garcia, L., Rodriguez, N., & Gomez, F. (2019). Lipid Biomarker and Carbon Stable Isotope Survey on the Dallol Hydrothermal System in Ethiopia. Astrobiology, 19(12), 1474-1489. Article.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 Cavalazzi, B., Barbieri, R., Gomez, F., Capaccioni, B., Olsson-Francis, K., Pondrelli, M., et al. (2019). The Dallol Geothermal Area, Northern Afar (Ethiopia)-An Exceptional Planetary Field Analog on Earth. Astrobiology, 19(4), 553-578. Review.
- ↑ Tadesse, S., Milesi, J. P., & Deschamps, Y. (2003). Geology and mineral potential of Ethiopia: a note on geology and mineral map of Ethiopia. Journal of African Earth Sciences, 36(4), 273-313. Article.
- ↑ Hovland, M., Rueslatten, H. G., Johnsen, H. K., Kvamme, B., & Kuznetsova, T. (2006). Salt formation associated with sub-surface boiling and supercritical water. Marine and Petroleum Geology, 23(8), 855-869. Article.
- ↑ Carniel, R., Jolis, E. M., & Jones, J. (2010). A geophysical multi-parametric analysis of hydrothermal activity at Dallol, Ethiopia. Journal of African Earth Sciences, 58(5), 812-819. Article.
- ↑ Hagos, M., Konka, B., & Ahmed, J. (2016). A preliminary Geological and Generalized Stratigraphy of Western Margin of Northern Afar Depression, Dallol Area, Northern Ethiopia. Momona Ethiopian Journal of Science, 8(1), 1-22. Article.
- ↑ 12.0 12.1 12.2 Gupta, R. S., Naushad, S., Fabros, R., & Adeolu, M. (2016). A phylogenomic reappraisal of family-level divisions within the class Halobacteria: proposal to divide the order Halobacteriales into the families Halobacteriaceae, Haloarculaceae fam. nov., and Halococcaceae fam. nov., and the order Haloferacales into the families, Haloferacaceae and Halorubraceae fam nov. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology, 109(4), 565-587. Article.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 Madigan, M.T., Bender, K.S., Buckley, D.H., Sattley, W.M., & Stahl, D.A. (2019). Brock Biology of Microorganisms. New York City, NY: Pearson.
- ↑ Grant, W. D., Gemmell, R. T., & McGenity, T. J. (1998). Halobacteria: the evidence for longevity. Extremophiles, 2(3), 279-287. Review.
- ↑ 15.0 15.1 15.2 15.3 Narasingarao, P., Podell, S., Ugalde, J. A., Brochier-Armanet, C., Emerson, J. B., Brocks, J. J., et al. (2012). De novo metagenomic assembly reveals abundant novel major lineage of Archaea in hypersaline microbial communities. Isme Journal, 6(1), 81-93. Article.
- ↑ Oren, A. (2014). Taxonomy of halophilic Archaea: current status and future challenges. Extremophiles, 18(5), 825-834. Review.
- ↑ 17.0 17.1 17.2 17.3 Aouad, M., Taib, N., Oudart, A., Lecocq, M., Gouy, M., & Brochier-Armanet, C. (2018). Extreme halophilic archaea derive from two distinct methanogen Class II lineages. Molecular Phylogenetics and Evolution, 127, 46-54. Article.
- ↑ 18.0 18.1 18.2 Hedlund, B. P., Reysenbach, A. L., Huang, L. Q., Ong, J. C., Liu, Z. Z., Dodsworth, J. A., et al. (2015). Isolation of diverse members of the Aquificales from geothermal springs in Tengchong, China. Frontiers in Microbiology, 6, 8. Article.
- ↑ 19.0 19.1 19.2 Nesbo, C. L., Bradnan, D. M., Adebusuyi, A., Dlutek, M., Petrus, A. K., Foght, J., et al. (2012). Mesotoga prima gen. nov., sp nov., the first described mesophilic species of the Thermotogales. Extremophiles, 16(3), 387-393. Article.
- ↑ Zhaxybayeva, O., Swithers, K. S., Lapierre, P., Fournier, G. P., Bickhart, D. M., Deboy, R. T., et al. (2009). On the chimeric nature, thermophilic origin, and phylogenetic placement of the Thermotogales. Proceedings of the National Academy of Sciences of the United States of America, 106(14), 5865-5870. Article.
- ↑ Segerer, A., Langworthy, T. A., & Stetter, K. O. (1988). Thermoplasma-Acidophilym and Thermoplasma-Volcanium sp-nov from solfatara fields. Systematic and Applied Microbiology, 10(2), 161-171. Article.
- ↑ Mesbah, N. M., & Wiegel, J. (2012). Life under Multiple Extreme Conditions: Diversity and Physiology of the Halophilic Alkalithermophiles. Applied and Environmental Microbiology, 78(12), 4074-4082. Review.
Authored for Earth 373 Microbial Ecology, taught by Magdalena Osburn, 2020, NU Earth Page.
