User:Synthetic Biology: Synthesizing Petroleum Replica Fuel: Difference between revisions
Jon.s.frew (talk | contribs) No edit summary |
Jon.s.frew (talk | contribs) No edit summary |
||
| Line 2: | Line 2: | ||
=Introduction= | =Introduction= | ||
The field of [http://en.wikipedia.org/wiki/Synthetic_biology synthetic biology] combines hierarchical design strategies derived from electrical and mechanical engineering with genetic manipulations of microorganisms to generate artificial enzymatic pathways[[#References|[1]]]. Strategies begin with an initial examination of the basic set of biological building blocks needed to develop systems of increasing complexity to enhance specific cellular outputs[[#References|[2]]]. Researchers can design and implement unique synthetic pathways, selecting from an expansive array of microbial enzymes[1]. Synthetic fuels must share similar chemical components as retail transport fuels which are composed of hydrocarbons (n-alkanes) of variable length, branched hydrocarbons (iso-alkanes), and unsaturated hydrocarbons (n-alkenes)[[#References|[3]]]. Designing and generating a microbial system aimed at synthesizing components of identical composition to petroleum fuel, eliminates several restricting factors limiting the success of alternative biofuels[[#References|[3]]]. | The field of [http://en.wikipedia.org/wiki/Synthetic_biology synthetic biology] combines hierarchical design strategies derived from electrical and mechanical engineering with genetic manipulations of microorganisms to generate artificial enzymatic pathways[[#References|[1]]]. Strategies begin with an initial examination of the basic set of biological building blocks needed to develop systems of increasing complexity to enhance specific cellular outputs[[#References|[2]]]. Researchers can design and implement unique synthetic pathways, selecting from an expansive array of microbial enzymes[1]. Synthetic fuels must share similar chemical components as retail transport fuels which are composed of hydrocarbons (n-alkanes) of variable length, branched hydrocarbons (iso-alkanes), and unsaturated hydrocarbons (n-alkenes)[[#References|[3]]]. Designing and generating a microbial system aimed at synthesizing components of identical composition to petroleum fuel, eliminates several restricting factors limiting the success of alternative biofuels[[#References|[3]]]. | ||
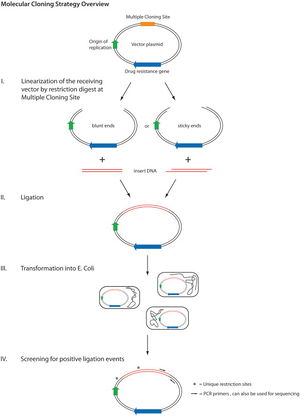
[[File:Molecular Cloning Diagram.jpeg|300px|thumb|right| Figure 1. An illustration outlining the basics of molecular cloning. | [[File:Molecular Cloning Diagram.jpeg|300px|thumb|right| Figure 1. An illustration outlining the basics of molecular cloning. | ||
Cited from: 12. Lessard JC. Molecular cloning. Methods in enzymology. 2013;529:85.]] | Cited from: 12. Lessard JC. Molecular cloning. Methods in enzymology. 2013;529:85.]] | ||
=Basics of Molecular Cloning: Synthetic Gene Expression= | |||
The growth of synthetic biology over the last decade is highly attributed to recent improvements in expression plasmid construction via [http://en.wikipedia.org/wiki/Molecular_cloning molecular cloning] techniques providing researchers with the ability to modulate an organism’s gene expression profile with precision and accuracy[[#References|[4]]]. Performing these alterations requires advanced molecular engineering beginning with sequence optimization of a particular protein, these sequences are then generated and amplified via [http://en.wikipedia.org/wiki/Polymerase_chain_reaction polymerase chain reaction](PCR)[[#References|[3]]][[#References|[5]]]. Subsequent utilization of site specific [http://en.wikipedia.org/wiki/Restriction_enzyme restriction endonucleases] allows optimized genes to be excised from their original sequence forming restriction gene fragments(Fig. 1)[[#References|[4]]]. Plasmids are selected with complementary restriction sites allowing the same restriction endonucleases to be used to linearize the expression plasmid[[#References|[4]]]. Mixing these two pools of genetic material together allows restriction gene fragments to be incorporated into the plasmids producing recombinant plasmids which can be permanently sealed via DNA ligase activity(Fig. 1)[[#References|[4]]]. Genes inducing antibiotic resistance are commonly included in expression vectors to select for successful uptake(Fig. 1)[[#References|[4]]]. In addition, plasmid restriction sites are often embedded within a colour indicator gene providing a means of quantifying successful plasmid recombination[[#References|[4]]]. Treatment with antibiotics selects for antibiotic resistant cells containing the desired plasmid, while colour associated cell sorting can distinguish cells possessing recombinant plasmids[[#References|[4]]]. Expression plasmids can then be cloned into specialized chemically competent [http://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli](Fig. 1) that can be propagated, frozen, or utilised for future experiments[[#References|[3]]]. | |||
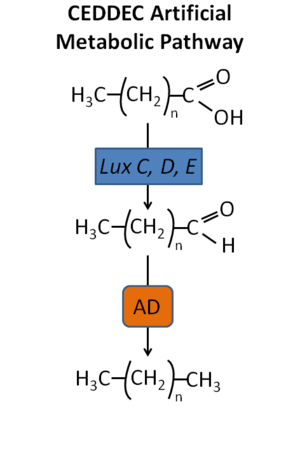
[[File:Original_Figure_CEDDEC.png|300px|thumb|right| Figure 2. Foundational groundwork outlining the basics of the CEDDEC artificial alkane synthesis pathway. 2013;529:85.]] | |||
=Components of Synthetic Alkane Synthesis= | |||
Designing an efficient metabolic system capable of generating a synthetic petroleum product requires a critical assessment of microorganisms capable of alkane synthesis, two key enzymes necessary for synthesis of alkanes from fatty acid include an aldehyde decarbonylase (AD) and a fatty acid reductase (FAR) complex[[#References|[3]]]. The cyanobacteria [http://en.wikipedia.org/wiki/Nostoc_punctiforme Nostoc punctiforme] produces a soluble AD which catalyzes the decarbonylation of long chain fatty aldehydes into alkanes[[#References|[6]]] (Fig. 2). This component represents a key final step for the de vovo synthesis of alkanes or alkenes. Working backwards, it is also fundamental to generate alkane synthesis precursors from the fatty acid (FA) pool[[#References|[3]]]. The bioluminescent bacteria [http://en.wikipedia.org/wiki/Photorhabdus_luminescens Photorhabdus luminescens], possesses a luxCDE operon forming a FAR complex required for synthesis of long chain fatty aldehydes from FA[[#References|[7]]]. These gene products can be incorporated together as key components of an alkane synthesis pathway (Fig. 2)[[#References|[3]]]. | |||
Subsequent considerations must address the system’s ability to create endogenous variation within the FA pool in order to match the variable hydrocarbon components of petroleum[[#References|[3]]]. Several gram positive organisms possess the ability to produce branched FA from branched amino acids[[#References|[3]]]. [http://en.wikipedia.org/wiki/Bacillus_subtilis Bacillus subtilis], in particular possesses the required B-ketoacyl-ACP synthase III (FabH2 KAS III) specific to branched chain precursors as well as a branched-chain α-keto acid dehydrogenase (BCKD) complex[[#References|[8]]][[#References|[9]]]. These complexes allow endogenous branched carbon chain precursors to be incorporated into the FA pool for variable alkane synthesis[[#References|[3]]]. | |||
==Alkane Synthesis: Synthetic Metabolic Pathway== | |||
Researchers at the University of Exeter cloned the aforementioned genes into various E.coli models generating synthetic organisms capable of alkane synthesis from a variable fatty acid pool[[#References|[3]]]. The initial co-expression of P.luminescens FAR complex consisting of luxC, D, and E genes in conjunction with the AD gene of N.punctiforme (total construct termed CEDDEC) resulted in significantly greater levels of the hydrocarbons: tridecane, pentadecane, pentadecene, hexadecene, heptadecane, and heptadecene when compared to the wild type N.punctiforme production[[#References|[3]]]. These initial alkane products share common chemical characteristics with constituents of diesel and aviation fuels[[#References|[3]]]. Supplementary experiments sought to manipulate the endogenous FA pool with the aim of introducing de novo diversification of alkane products[[#References|[3]]]. | |||
Branched hydrocarbons are common components of petroleum based fuels. An initial assessment was performed to determine the alterations to the FA pool by expression of B.subtilis derived BCKD complex and FabH2 KAS III[[#References|[3]]]. Results revealed an increase in chain variation with the novel synthesis of methyl tetradecanoic acid and methyl hexadecanoic acid[[#References|[3]]]. They next simultaneously expressed the BCKD complex and FabH2 KAS III specific to branched precursors with the already developed CEDDEC construct[[#References|[3]]]. The combination experiment displayed an overall decrease in alkane production, but still maintained the wide range of hydrocarbon production achieved previously[[#References|[3]]]. This result can be attributed an overall net reduction on recombinant protein expression levels compared to CEDDEC alone[[#References|[3]]]. | |||
=Future Directions & Commercial Applications= | |||
The transportation industry accounts for 60% of petroleum based fuel consumption, making it the second largest global contributor of greenhouse gas emissions[[#References|[3]]]. Today’s current alternative biofuels are primarily alcohol and biodiesel based; their efficacy is limited by several key factors[[#References|[11]]][[#References|[12]]]. With today’s current infrastructure and engine specifications biodiesel based fuels can only be supported with a blend ratio of 10-20% biodiesel to 80-90% petroleum distillate, known as the “blend-wall” [[#References|[12]]]. The presented artificial pathway circumvents the “blend wall” limitation of current biofuels, producing fossil fuel replicas via endogenous alkane synthesis[[#References|[3]]]. These findings represent a major step in advancing technologies supporting production of microbial derived fuel sources[[#References|[3]]]. Further studies exploring adaptations of the presented model may include alternative engineering strategies aimed at enhancing components of the FAR complex as well as eliminating product limiting side reactions[[#References|[3]]]. Moreover, the progression from bench top to the development of commercial applications will require further manipulation of endogenous FA synthesis pathways, alterations of host cell biology, optimization of fermentation protocols, and improved methods of fuel extraction[[#References|[3]]]. Future innovations within synthetic biology aimed at developing the renewable energy sector will be driven by the necessity of human self-sustainability. | |||
[[ | |||
=References= | =References= | ||
Revision as of 06:55, 22 November 2013
Introduction
The field of synthetic biology combines hierarchical design strategies derived from electrical and mechanical engineering with genetic manipulations of microorganisms to generate artificial enzymatic pathways[1]. Strategies begin with an initial examination of the basic set of biological building blocks needed to develop systems of increasing complexity to enhance specific cellular outputs[2]. Researchers can design and implement unique synthetic pathways, selecting from an expansive array of microbial enzymes[1]. Synthetic fuels must share similar chemical components as retail transport fuels which are composed of hydrocarbons (n-alkanes) of variable length, branched hydrocarbons (iso-alkanes), and unsaturated hydrocarbons (n-alkenes)[3]. Designing and generating a microbial system aimed at synthesizing components of identical composition to petroleum fuel, eliminates several restricting factors limiting the success of alternative biofuels[3].
Basics of Molecular Cloning: Synthetic Gene Expression
The growth of synthetic biology over the last decade is highly attributed to recent improvements in expression plasmid construction via molecular cloning techniques providing researchers with the ability to modulate an organism’s gene expression profile with precision and accuracy[4]. Performing these alterations requires advanced molecular engineering beginning with sequence optimization of a particular protein, these sequences are then generated and amplified via polymerase chain reaction(PCR)[3][5]. Subsequent utilization of site specific restriction endonucleases allows optimized genes to be excised from their original sequence forming restriction gene fragments(Fig. 1)[4]. Plasmids are selected with complementary restriction sites allowing the same restriction endonucleases to be used to linearize the expression plasmid[4]. Mixing these two pools of genetic material together allows restriction gene fragments to be incorporated into the plasmids producing recombinant plasmids which can be permanently sealed via DNA ligase activity(Fig. 1)[4]. Genes inducing antibiotic resistance are commonly included in expression vectors to select for successful uptake(Fig. 1)[4]. In addition, plasmid restriction sites are often embedded within a colour indicator gene providing a means of quantifying successful plasmid recombination[4]. Treatment with antibiotics selects for antibiotic resistant cells containing the desired plasmid, while colour associated cell sorting can distinguish cells possessing recombinant plasmids[4]. Expression plasmids can then be cloned into specialized chemically competent Escherichia coli(Fig. 1) that can be propagated, frozen, or utilised for future experiments[3].
Components of Synthetic Alkane Synthesis
Designing an efficient metabolic system capable of generating a synthetic petroleum product requires a critical assessment of microorganisms capable of alkane synthesis, two key enzymes necessary for synthesis of alkanes from fatty acid include an aldehyde decarbonylase (AD) and a fatty acid reductase (FAR) complex[3]. The cyanobacteria Nostoc punctiforme produces a soluble AD which catalyzes the decarbonylation of long chain fatty aldehydes into alkanes[6] (Fig. 2). This component represents a key final step for the de vovo synthesis of alkanes or alkenes. Working backwards, it is also fundamental to generate alkane synthesis precursors from the fatty acid (FA) pool[3]. The bioluminescent bacteria Photorhabdus luminescens, possesses a luxCDE operon forming a FAR complex required for synthesis of long chain fatty aldehydes from FA[7]. These gene products can be incorporated together as key components of an alkane synthesis pathway (Fig. 2)[3].
Subsequent considerations must address the system’s ability to create endogenous variation within the FA pool in order to match the variable hydrocarbon components of petroleum[3]. Several gram positive organisms possess the ability to produce branched FA from branched amino acids[3]. Bacillus subtilis, in particular possesses the required B-ketoacyl-ACP synthase III (FabH2 KAS III) specific to branched chain precursors as well as a branched-chain α-keto acid dehydrogenase (BCKD) complex[8][9]. These complexes allow endogenous branched carbon chain precursors to be incorporated into the FA pool for variable alkane synthesis[3].
Alkane Synthesis: Synthetic Metabolic Pathway
Researchers at the University of Exeter cloned the aforementioned genes into various E.coli models generating synthetic organisms capable of alkane synthesis from a variable fatty acid pool[3]. The initial co-expression of P.luminescens FAR complex consisting of luxC, D, and E genes in conjunction with the AD gene of N.punctiforme (total construct termed CEDDEC) resulted in significantly greater levels of the hydrocarbons: tridecane, pentadecane, pentadecene, hexadecene, heptadecane, and heptadecene when compared to the wild type N.punctiforme production[3]. These initial alkane products share common chemical characteristics with constituents of diesel and aviation fuels[3]. Supplementary experiments sought to manipulate the endogenous FA pool with the aim of introducing de novo diversification of alkane products[3].
Branched hydrocarbons are common components of petroleum based fuels. An initial assessment was performed to determine the alterations to the FA pool by expression of B.subtilis derived BCKD complex and FabH2 KAS III[3]. Results revealed an increase in chain variation with the novel synthesis of methyl tetradecanoic acid and methyl hexadecanoic acid[3]. They next simultaneously expressed the BCKD complex and FabH2 KAS III specific to branched precursors with the already developed CEDDEC construct[3]. The combination experiment displayed an overall decrease in alkane production, but still maintained the wide range of hydrocarbon production achieved previously[3]. This result can be attributed an overall net reduction on recombinant protein expression levels compared to CEDDEC alone[3].
Future Directions & Commercial Applications
The transportation industry accounts for 60% of petroleum based fuel consumption, making it the second largest global contributor of greenhouse gas emissions[3]. Today’s current alternative biofuels are primarily alcohol and biodiesel based; their efficacy is limited by several key factors[11][12]. With today’s current infrastructure and engine specifications biodiesel based fuels can only be supported with a blend ratio of 10-20% biodiesel to 80-90% petroleum distillate, known as the “blend-wall” [12]. The presented artificial pathway circumvents the “blend wall” limitation of current biofuels, producing fossil fuel replicas via endogenous alkane synthesis[3]. These findings represent a major step in advancing technologies supporting production of microbial derived fuel sources[3]. Further studies exploring adaptations of the presented model may include alternative engineering strategies aimed at enhancing components of the FAR complex as well as eliminating product limiting side reactions[3]. Moreover, the progression from bench top to the development of commercial applications will require further manipulation of endogenous FA synthesis pathways, alterations of host cell biology, optimization of fermentation protocols, and improved methods of fuel extraction[3]. Future innovations within synthetic biology aimed at developing the renewable energy sector will be driven by the necessity of human self-sustainability.
References
(1) Chiarabelli C, Stano P, Anella F, Carrara P, Luisi PL. Approaches to chemical synthetic biology. FEBS Letters. 2012
(2) Pleiss J. The promise of synthetic biology. Applied microbiology and biotechnology. 2006;73:735-739.
(3) Howard, T. P., Aves, S. J., Love, J., Middelhaufe, S., Moore, K., Edner, C., Smirnoff, N. (2013). Synthesis of customized petroleum-replica fuel molecules by targeted modification of free fatty acid pools in escherichia coli. Proceedings of the National Academy of Sciences of the United States of America,110(19), 7636.
(4) Lessard JC. Molecular cloning. Methods in enzymology. 2013;529:85.
(5) Tefferi A. Genomics Basics: DNA Structure, Gene Expression, Cloning, Genetic Mapping, and Molecular Tests. Seminars in Cardiothoracic and Vascular Anesthesia. 2006;10:282-290.
(6) Das D, Eser BE, Han J, Sciore A, Marsh ENG (2011) Oxygen-independent decarbonylation of aldehydes by cyanobacterial aldehyde decarbonylase: A new reaction of diiron enzymes. Angew Chem Int Ed 50:7148–7152
(7) Winson MK, Swift S, Hill PJ, et al. Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS microbiology letters. 1998;163:193-202.
(8) Choi KH, Heath RJ, Rock CO (2000) β-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis.J Bacteriol 182(2):365–370.
(9) Namba Y, Yoshizawa K, Ejima A, Hayashi T, Kaneda T. Coenzyme A- and nicotinamide adenine dinucleotide-dependent branched chain alpha-keto acid dehydrogenase. I. Purification and properties of the enzyme from Bacillus subtilis. The Journal of biological chemistry. 1969;244:4437.
(10) International Energy Agency (2011) Key World Statistics (Paris, France).
(11) Yuksel F, Yuksel B (2004) The use of ethanol-gasoline blend as a fuel in an SI engine. Renew Energy 29:1181–1191.
(12) National Renewable Energy Laboratory (2009) Biodiesel Handling and User Guide (Oak Ridge, TN: US Department of Commerce), 4th Ed.