User talk:Pmedina0098: Difference between revisions
Pmedina0098 (talk | contribs) |
Pmedina0098 (talk | contribs) |
||
| Line 13: | Line 13: | ||
==Polar Charged Residues== | ==Polar Charged Residues== | ||
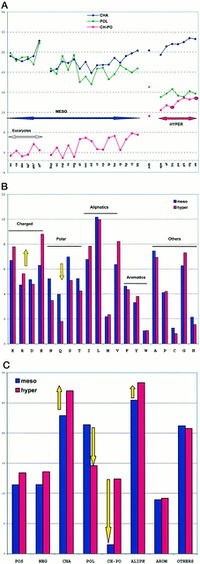
<br>The replacement of polar noncharged residues by charged ones constitutes a major stabilization mechanism in the proteins of hyperthermophilic organisms. Residue changes allow the stabilization of proteins through ion bonds. | <br>[[Image:polar charged residues.jpg|thumb|200px|right|Amino acid content variations within 30 genomes. Cambillau and Claverie 2000]] The replacement of polar noncharged residues by charged ones constitutes a major stabilization mechanism in the proteins of hyperthermophilic organisms. Residue changes allow the stabilization of proteins through ion bonds. Part A is a plot of the sum of the percentages of charged amino acids (Lys, Arg, Asp, Glu; CHA, blue), polar noncharged amino acids (Asn, Gln, Ser, Thr; POL, green), and of the difference of the two values (CH-POL, red). The mesophiles and hyperthermophiles are identified by MESO and HYPER, respectively. Part B is a plot of the percentages of the various amino acids in mesophiles (blue) and hyperthermophiles (red). Part C, shows a plot of the sum of percentages of the various amino acid classes in mesophiles (blue) and hyperthermophiles (red). The proteome analysis in amino acid classes indicated that hyperthermophilicity is characterized by a sharp increase of charged residues, Lys and Glu, at the expense of polar noncharged residues, mainly Gln. The difference between charged and polar noncharged amino acids (Ch-Po) is the best indicator of an organism’s lifestyle. A threshold is observed around 10% for extremeophiles Ch-Po values, while a higher limit of 5% characterizes mesophiles.<br> | ||
<br>The charged polar amino acid content is a genomic signature that can give strong indications to an organisms growth environment. The advantage of this characteristic comes from the increased stability of coulombic interactions with temperature. In adition to this, in most structures of hyperthermophilic proteins, the existence of long chains of ion pairs provide cooperative stabilization (Mandrich). | <br>The charged polar amino acid content is a genomic signature that can give strong indications to an organisms growth environment. The advantage of this characteristic comes from the increased stability of coulombic interactions with temperature. In adition to this, in most structures of hyperthermophilic proteins, the existence of long chains of ion pairs provide cooperative stabilization (Mandrich). | ||
<br> | <br> | ||
Revision as of 22:31, 25 March 2013
Introduction
The National Center for Biotechnology Inforation (NCBI) Microbial Genome Project Database uses five terms to categorize the temperature range an organism grows at, where cryophilic refers to –30° to –2°C, psychrophilic refers to –1° to +10°C, mesophilic refers to +11° to +45°C, thermophilic refers to +46° to 75°C, and hyperthermophilic refers to above +75°C.
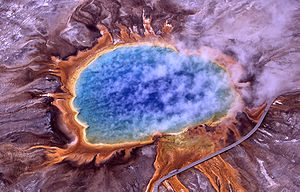
High temperatures can often denature enzymes and proteins that are vital to an organisms survival. Unlike these types of organisms, thermophiles can survive and thrive at very high temperatures. They found in geothermally heated regions of the Earth like deep-sea hydrothermal vents and the hot springs of Yellowstone National Park. The investigation of thermophilic physiology poses very promising and intriguing contributions to the scientific community. For one, some of the enzymes used in molecular biology, like DNA polymerases, have derived from investigating heat-stable enzymes. In addition, astrobiologists look to understand the structural and genomic correlates of hyoerthermostability in order to give indication to what life may look like on planets hotter than ours.
Research has suggested physical adaptations that allow thermophiles to remain functional and alive at high temperatures. First, increasing the number of salt bridges is a driving force for enhancement of the thermotolerance of proteins from hyperthermophilic microorganisms. Second, research suggests that the replacement of polar noncharged resides by charged ones constitutes a major stabilization mechanisms in the proteins of hyperthermophilic organisms. Third, thermophilic protein sequences are more likely than their mesophilic homologs to have deletions in exposed loop regions.
Salt Bridges
The optimization of electrostatic interactions by increasing the number of salt bridges is a driving force for enhancement of the thermotolerance of proteins from hyperthermophilic microorganisms. This trend is less evident in thermophilic organisms and absent from mesophile-derived proteins. A salt bridge is a combination of two noncovalent interactions, hydrogen bonding and electrostatic interaction. Salt bridges often occur between groups distant in the protein sequence and form cross-links that stabilize tertiary structure. This interaction can increase the kinetic barrier towards thermal inactivation or thermal unfolding.
The table above shows the number of salt bridges in select thermo- and hyperthermophilic organisms. Ns indicates the number of salt bridges, Nr represents the number of salt bridges statistically expected for that protein structure, and Topt represents the temperature of optimal growth for the protein. Proteins from hyperthermophilic organisms are characterized by an increased number of ion pairs with respect to the statistical expectance and/or the number of ion-pairs in their mesophilic counterparts. This finding suggests that electrostatic interactions are a principal factor responsible for the elevation of the melting temperature of proteins from hyperthermophilic organisms.
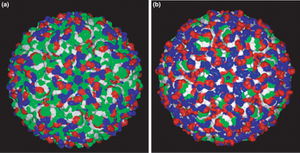
The figure and table above show that charged residues in the enzyme from Aquifex aeolicus replace most of the polar residues in the Bacillus subtilis enzyme. Red represents negatively charged residues, blue represents positively charged, green represents polar, and white represents non-polar. Specifically, the number of ion pairs in the protein from Aquifex is increased by >90%. Melting point assessments are often employed in thermostability studies in order to examine the effect of structural changes. In this case, the corresponding change in melting temperature when B. subtilis to A. aeolicus was about 27°C.
Molecular dynamics calculations on a prototypical ion-pair model system have suggested that a sizeable energy barrier exists for the solvation of a salt bridge and that the height of this barrier increases with temperature. Interestingly, a similar barrier is not seen with isosteric hydrophobic groups.
Polar Charged Residues
The replacement of polar noncharged residues by charged ones constitutes a major stabilization mechanism in the proteins of hyperthermophilic organisms. Residue changes allow the stabilization of proteins through ion bonds. Part A is a plot of the sum of the percentages of charged amino acids (Lys, Arg, Asp, Glu; CHA, blue), polar noncharged amino acids (Asn, Gln, Ser, Thr; POL, green), and of the difference of the two values (CH-POL, red). The mesophiles and hyperthermophiles are identified by MESO and HYPER, respectively. Part B is a plot of the percentages of the various amino acids in mesophiles (blue) and hyperthermophiles (red). Part C, shows a plot of the sum of percentages of the various amino acid classes in mesophiles (blue) and hyperthermophiles (red). The proteome analysis in amino acid classes indicated that hyperthermophilicity is characterized by a sharp increase of charged residues, Lys and Glu, at the expense of polar noncharged residues, mainly Gln. The difference between charged and polar noncharged amino acids (Ch-Po) is the best indicator of an organism’s lifestyle. A threshold is observed around 10% for extremeophiles Ch-Po values, while a higher limit of 5% characterizes mesophiles.
The charged polar amino acid content is a genomic signature that can give strong indications to an organisms growth environment. The advantage of this characteristic comes from the increased stability of coulombic interactions with temperature. In adition to this, in most structures of hyperthermophilic proteins, the existence of long chains of ion pairs provide cooperative stabilization (Mandrich).
This finding implies the existence of global structural features associated with hyperthermostability common to thermophilic Bacteria and Achaea.
Loop Deletions
Sequence alignments to proteins of known structure indicate that thermophilic sequences are more likely than their mesophilic homologs to have deletions in exposed loop regions. Loops were identified as the intervening regions between transmembrane segments (Merueio). This finding indicates a general evolutionary strategy for increasing thermostability and is thought to be a mechanism for reducing unfolded state entropy (Thomson). In order to investigate loop length, it would be ideal for
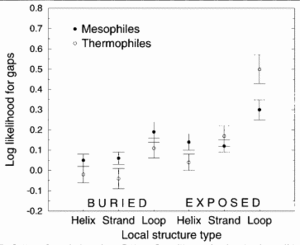
The figure demonstrates that thermophilic sequences have an increased propensity for deletions in exposed loops. Here, three types of secondary structure, helix, strand, and loop, were considered. The figure depicts the structural propensities for gaps found in alignments between proteins of known structure and their mesophilic or thermophilic homologs. Propensities less than 0 for a given structure type indicate that fewer gaps are associate with that structure type than random expectation while higher propensities indicate that more gaps are associated. Here, the propensities were averaged over homologs for each protein of known structure. This figure suggests that exposed loops of thermophiles are the only structura elements having significantly greater gaps compared to mesophiles.
Deletion of the exposed loop residues would decrease the unfolding entropy while having minimal impact on the enthalpy of unfolding. A full thermodynamic argument for this conclusion can be found in Thomson and Eisenberg 1999.
GC Content
Since the GC pair is bound by three hydrogen bonds while the adenine-thymine (AT) pair is bound by two hydrogen bonds, it is expected that organisms growing at higher temperature would have a higher proportion of GC than AT pairs. Hao and Wu found the GC content levels of the coding/non-coding regions of certain genes are highly likely to be correlated with the temperature range conditions of prokaryotic organisms. Four genes were consistently identified as correlated with the temperature range condition: K01251 (adenosylhomocysteinase), K03724 (DNA repair and recombination proteins), K07588 (LAO/AO transport system kinase), and K09122 (hypothetical protein). When these four genomic regions were used to predict the temperature range condition of an organism, the prediction accuracy was 84.52% for complete genomes, 84.09% for the in-progress genomes, and 82.70% for the metagenomes. Considering that these four genes only account for less than 1% of all the 413 genomic regions potentially correlated with the temperature range condition but can to a great extent retain the prediction accuracy, we may interpret these four genomic regions as the core of the temperature range-correlated.
To further validate the relationship correlation between GC content and
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
Edited by (your name here), a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.