User talk:Pmedina0098
Introduction
The National Center for Biotechnology Inforation (NCBI) Microbial Genome Project Database uses five terms to categorize the temperature range an organism grows at, where <a href="http://en.wikipedia.org/wiki/Cryophilic">cryophilic</a> refers to –30° to –2°C, psychrophilic refers to –1° to +10°C, mesophilic refers to +11° to +45°C, thermophilic refers to +46° to 75°C, and hyperthermophilic refers to above +75°C.
High temperatures can often denature enzymes and proteins that are vital to an organisms survival. Unlike these types of organisms, thermophiles can survive and thrive at very high temperatures. They found in geothermally heated regions of the Earth like deep-sea hydrothermal vents and the hot springs of Yellowstone National Park. The investigation of thermophilic physiology poses very promising and intriguing contributions to the scientific community. For one, some of the enzymes used in molecular biology, like DNA polymerases, have derived from investigating heat-stable enzymes. In addition, astrobiologists look to understand the structural and genomic correlates of hyoerthermostability in order to give indication to what life may look like on planets hotter than ours.
Research has suggested physical adaptations that allow thermophiles to remain functional and alive at high temperatures. First, increasing the number of salt bridges is a driving force for enhancement of the thermotolerance of proteins from hyperthermophilic microorganisms. Second, research suggests that the replacement of polar noncharged resides by charged ones constitutes a major stabilization mechanisms in the proteins of hyperthermophilic organisms. Third, thermophilic protein sequences are more likely than their mesophilic homologs to have deletions in exposed loop regions.
Salt Bridges
The optimization of electrostatic interactions by increasing the number of salt bridges is a driving force for enhancement of the thermotolerance of proteins from hyperthermophilic microorganisms. This trend is less evident in thermophilic organisms and absent from mesophile-derived proteins. A salt bridge is a combination of two noncovalent interactions, hydrogen bonding and electrostatic interaction. Salt bridges often occur between groups distant in the protein sequence and form cross-links that stabilize tertiary structure. This interaction can increase the kinetic barrier towards thermal inactivation or thermal unfolding.
The table above shows the number of salt bridges in select thermo- and hyperthermophilic organisms. Ns indicates the number of salt bridges, Nr represents the number of salt bridges statistically expected for that protein structure, and Topt represents the temperature of optimal growth for the protein. Proteins from hyperthermophilic organisms are characterized by an increased number of ion pairs with respect to the statistical expectance and/or the number of ion-pairs in their mesophilic counterparts. This finding suggests that electrostatic interactions are a principal factor responsible for the elevation of the melting temperature of proteins from hyperthermophilic organisms.
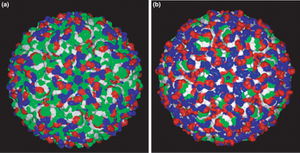
The figure and table above show that charged residues in the enzyme from Aquifex aeolicus replace most of the polar residues in the Bacillus subtilis enzyme. Red represents negatively charged residues, blue represents positively charged, green represents polar, and white represents non-polar. Specifically, the number of ion pairs in the protein from Aquifex is increased by >90%. Melting point assessments are often employed in thermostability studies in order to examine the effect of structural changes. In this case, the corresponding change in melting temperature when B. subtilis to A. aeolicus was about 27°C.
Molecular dynamics calculations on a prototypical ion-pair model system have suggested that a sizeable energy barrier exists for the solvation of a salt bridge and that the height of this barrier increases with temperature. Interestingly, a similar barrier is not seen with isosteric hydrophobic groups.
Polar Charged Residues
Include some current research in each topic, with at least one figure showing data.
Loop Deletions
Include some current research in each topic, with at least one figure showing data.
GC Content
Include some current research in each topic, with at least one figure showing data.
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
Edited by (your name here), a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.