Venezuelan equine encephalitis virus: Difference between revisions
| (4 intermediate revisions by the same user not shown) | |||
| Line 20: | Line 20: | ||
==Description and Significance== | ==Description and Significance== | ||
Venezuelan equine encephalitis virus (VEEV) is an important animal and human pathogen found only in the Americas (the New World). VEEV is actually a complex of 7 different species as well as multiple subtypes and varieties [ | Venezuelan equine encephalitis virus (VEEV) is an important animal and human pathogen found only in the Americas (the New World). VEEV is actually a complex of 7 different species as well as multiple subtypes and varieties [3]. Depending on the subtype, VEEV maintains either an enzootic or an epizootic life cycle. Enzootic species sustain a chain of transmission between a rodent reservoir and mosquito vector. Epizootic species cause epidemics in horses and other animals of agricultural importance. Most human cases of VEEV result from spill-over into the human population from an epizootic outbreak. Human are only incidental and usually dead-end hosts. | ||
<br><br> | <br><br> | ||
In the 1960's VEEV was weaponized by the U.S. and the U.S.S.R.[ | In the 1960's VEEV was weaponized by the U.S. and the U.S.S.R. [4]. The virus is considered a category B select bioterrorism threat by the U.S. government because it causes only moderate morbidity and low mortality in humans but severe morbidity and mortality in animals. | ||
<br><br> | <br><br> | ||
The virus has a biosafety level | The virus has a biosafety level of 3 (BSL 3). It can only be worked with inside of specially equipped laboratories designed to deal with BSL 3 agents [5]. although VEEV is transmitted by mosquitoes in nature it is a very good aerosol. The virus has been implicated in more than 150 cases of accidental laboratory exposures that were not associated with cuts or needle pricks [6]. | ||
<br><br> | <br><br> | ||
VEEV is an arbovirus (arthropod-virus), a general term for a virus that is transmitted via a hematophagous arthropod vector such as mosquitoes, flies and ticks. To be considered a true arbovirus there must be replication of the virus within the vector and VEEV is no exception [3]. | |||
VEEV is an arbovirus (arthropod-virus), a general term | |||
<br><br> | <br><br> | ||
The genus alphaviruses is included in the general group of Alphaviruses cause arthritic and encephalitic disease in mammals. This group includes the pathogens O'nyong'nyong virus, Chikungunya virus, Western equine encephalitis virus, Eastern equine encephalitis virus, and Venezuelan equine encephalitis. Alphaviruses are not only important human and animal pathogens but have also been used as model systems for the study of enveloped virus structure and as viral vectors in gene therapy research [2]. | |||
==Genome Structure== | ==Genome Structure== | ||
| Line 39: | Line 37: | ||
[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TD4-4XG52JN-M&_user=7774802&_coverDate=11%2F05%2F2009&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000062877&_version=1&_urlVersion=0&_userid=7774802&md5=8024782bd6bb05c40cdbb28a254145b9&searchtype=a].]] | [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TD4-4XG52JN-M&_user=7774802&_coverDate=11%2F05%2F2009&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000062877&_version=1&_urlVersion=0&_userid=7774802&md5=8024782bd6bb05c40cdbb28a254145b9&searchtype=a].]] | ||
VEEV has a non-segmented 11.4 kb (+) sense ssRNA genome with a poly (A) tail. The genome contains two reading frames that encode for two different polyproteins. Two-thirds of the genome starting from the 5’ end encode for a polyprotein that contains the four nonstructural proteins nsP1, nsP2, nsP3, and nsP4 (viral RNA-dependent RNA polymerase) that are required for genome replication. The last | VEEV has a non-segmented 11.4 kb (+) sense ssRNA genome with a poly (A) tail. The genome contains two reading frames that encode for two different polyproteins. Two-thirds of the genome starting from the 5’ end encode for a polyprotein that contains the four nonstructural proteins nsP1, nsP2, nsP3, and nsP4 (viral RNA-dependent RNA polymerase) that are required for genome replication. The last third of the genome encodes for a polyprotein with the capsid and envelope proteins CP, pE2, 6K, and E1 [7]. | ||
<br><br> | <br><br> | ||
The viral RNA itself contains four conserved sequence elements (CSEs) that interact with various viral and host proteins | The viral RNA itself contains four conserved sequence elements (CSEs) that interact with various viral and host proteins involved in the replication, transcription, and packaging of viral RNA. A small non-translated region at the beginning of the 5’ end of the genome (CSE1) appears to fold up into a stem-loop structure that acts as a promoter for (+) RNA strand replication from a (-) RNA template. CSE2 is found near the 5’ end as well but it is within the coding region for nsP1 and may be a promoter for the synthesis of (-) strand template RNA from genomic (+) strand RNA. CSE4, located just upstream of the poly (A) tail near the 3’ end appears to be involved in promoting the synthesis of template RNA as well. CSE3 is located between the two ORFs and is required for transcription of subgenomic RNA (smaller sections of mRNA that encode for only part of the genome). Just upstream Near CSE4 there are also repeated sequence elements 40-60-nt in length that bind to host proteins involved in the translation of viral RNA [2]. | ||
==Virion Structure of Venezuelan equine encephalitis virus== | ==Virion Structure of Venezuelan equine encephalitis virus== | ||
<br>Venezuelan equine encephalitis virus has an enveloped isohedral nucleocapsid. The capsid has a triangulation of t=4. Virions appear round and are 65-70 nm in diameter. The envelope surrounding the nucleocapsid | <br>Venezuelan equine encephalitis virus has an enveloped isohedral nucleocapsid. The capsid has a triangulation of t=4. Virions appear round and are 65-70 nm in diameter. The envelope surrounding the nucleocapsid is derived from host lipid bilayer embedded with approximately 240 copies of E1 and E2 glycoproteins. The glycoproteins form heterodimer spikes that link up in an isohedral lattice that also displays t=4 symmetry like the Nucleocapsid [2]. | ||
==Reproductive Cycle of Venezuelan equine encephalitis virus in a Host Cell== | ==Reproductive Cycle of Venezuelan equine encephalitis virus in a Host Cell== | ||
| Line 60: | Line 58: | ||
Once attached to the receptor the virus is endocytosed and the pH of the endosome drops and causes the E1-E2 dimer to destabilize and then trimerize with the late endosomal membrane. This trimerization allows for the fusion of the viral envelope with the endosome membrane and the eventual escape of the nucleocapsid into the cytoplasm. The exact mechanism of nucleocapsid uncoating in the cytoplasm is not known but may be related to some sort of destabilization by the initial drop in pH in the endosome. | Once attached to the receptor the virus is endocytosed and the pH of the endosome drops and causes the E1-E2 dimer to destabilize and then trimerize with the late endosomal membrane. This trimerization allows for the fusion of the viral envelope with the endosome membrane and the eventual escape of the nucleocapsid into the cytoplasm. The exact mechanism of nucleocapsid uncoating in the cytoplasm is not known but may be related to some sort of destabilization by the initial drop in pH in the endosome. | ||
<br><br> | <br><br> | ||
(+) Genomic RNA is translated using host ribosomes and the nonstructural polyprotein is processed and the resulting proteins form replication complexes within vacuoles derived from lysosomal and endosomal membranes | (+) Genomic RNA is translated using host ribosomes and the nonstructural polyprotein is processed and the resulting proteins form replication complexes within vacuoles derived from lysosomal and endosomal membranes. Replication complexes begin synthesis of (-) template RNA from which both genomic RNA and subgenomic RNA are transcribed. The subgenomic RNA only encodes for the structural polyprotein that contains envelope and capsid components. The capsid subunit CP autoproteolyses and self assembles into nucleocapsids in the cytoplasm and genomic RNA is encapsidated by an unknown mechanism [2]. | ||
<br><br> | <br><br> | ||
The remaining segment of polyprotein translocates into the ER. E1 and pE2 are folded and glycosylated in the ER and then | The remaining segment of polyprotein translocates into the ER. E1 and pE2 are folded and glycosylated in the ER and then taken to the golgi body where pE2 is cleaved to form E2 and E3. E3 seems to be involved with the dimerization between E1 and E2 but its exact role in viral replication has yet to be elucidated [2]. From the golgi the E1-E2 heterodimer is transported to the plasma membrane where E2 is required for nucleocapsid fusion with the plasma membrane and eventual viral budding. | ||
<br><br> | <br><br> | ||
During infection VEEV | During infection VEEV turns off cellular transcription and translation which leads to a decreased response from the innate immune system. Infection of the cell also initiates apoptosis by some unknown mechanism [2]. | ||
==Viral Ecology & Pathology== | ==Viral Ecology & Pathology== | ||
<br> | <br> | ||
In nature VEEV is only transmitted by mosquitoes but aerosol transmission has been observed in the | In nature VEEV is only transmitted by mosquitoes but aerosol transmission has been observed in the laboratory. Mosquitoes become infected after feeding on infected blood. The virus eventually escapes the gut of the insect and makes its way to the salivary glands where it replicates. The new host is infected when virus laden saliva is injected into the skin during feeding [3]. | ||
<br><br> | <br><br> | ||
VEEV can either sustain an enzootic or epizootic life cycle. Enzootic subtypes have a smaller host range | VEEV can either sustain an enzootic or epizootic life cycle. Enzootic subtypes have a smaller host range and are transmitted between reservoir rodent hosts by mosquitoes of the genus <I> Culex </I>. These subtypes (II-IV) rarely cause disease unless a susceptible human or horse ends up in the middle of the transmission cycle. In order for an enzootic strain to start an epizootic There must be changes in genotype as well as geographic cross-over between susceptible animals and mosquito vectors. This can occur when there is a dramatic change in mosquito habitat such as an unusually wet season that allows the insects to move beyond their normal geographic range. Susceptible hosts must be within geographic range of the vector swamps and forests (this often times occurs when land is cleared for agricultural use) [3]. | ||
<br><br> | <br><br> | ||
The epizootic subtypes of VEEV are IAC and IC. | The epizootic subtypes of VEEV are IAC and IC. They are responsible for most major human and equine (horse) epidemics. Epizootic subtypes arise from changes in the amino acid sequence of the E2 envelop protein. Even single amino acid substitutions can result in epizootic strains. E2 protein is responsible for binding to host receptors so changes in the structure of this protein affect host range which in turn allows for zoonotic outbreaks (epidemics in animals) that can incidentally involve humans [8]. Changes in E2 structure allow mosquitoes of the genera <I>Ochlerotatus</I> and <I>Psorphora</I> to transmit the disease to horses and other mammals that display high titres of virus in their blood (viremia). This viremia is important in maintaining an epidemic. Luckily for the animals that survive infection (mortality is high) the antibodies produced by the immune systems are strong enough to prevent reinfection. | ||
<br><br> | <br><br> | ||
VEEV in humans presents mostly as a flu-like illness and | VEEV in humans presents mostly as a flu-like illness and is often times missed because of a lack of surveillance and laboratory diagnosis. Disease mortality is less than 1% and (encephalitic) only 14% of cases involve the central nervous system. In fatal human cases there is edema, congestion, hemorrhages, meningitis, and encephalitis of the brain and spinal cord. The lungs, liver and lymphoid tissue are also ravaged by the virus [8]. | ||
<br><br> | <br><br> | ||
In horses, epizootic VEEV has very high mortality (50-90%). It causes swelling of the brain (encephalitis) that often-times | In horses, epizootic VEEV has very high mortality (50-90%). It causes swelling of the brain (encephalitis) that often-times result in fatal seizures [1]. | ||
==Vaccines and Treatments== | ==Vaccines and Treatments== | ||
<br> | <br> | ||
Currently treatment of VEEV infection is mostly supportive because there are no specific drugs for alphaviruses | Currently treatment of VEEV infection is mostly supportive because there are no specific drugs for alphaviruses. | ||
<br><br> | |||
There is currently a vaccine available for both humans and horses. The live attenuated vaccine known as TC-83 is a strain of VEEV that was passed 83 times in guinea pig heart cells [7]. There is also an inactivated form of the vaccine known as C-84 derived from the TC-83 strain. Currently only the C-84 vaccine is licensed for use in horses in the U.S. although countries such as Mexico and Colombia still produce the live vaccine for horses. In the U.S. only at risk military and laboratory personal are vaccinated with the TC-83 strain and some receive C-84 boosters if initial vaccination did not produce sufficient immunity. The vaccine does have side effects that ranged from mild to moderate and did not provided full protection of nonhuman primates challenged by aerosol exposure the route of transmission most likely if VEEV were to be used in a biological terrorist attack [7].<br> | |||
==References== | ==References== | ||
[1] Jose, J., J.E. Snyder, R.J. Kuhn. “A structural and functional perspective of alphavirus replication and | [1] Walton, T., E., Suchman. “Venezuelan Equine Encephalitis (VEE) Virus” American Society for Microbiology. MicrobeLibrary.org. http://www.microbelibrary.org/asmonly/details.asp?id=2407 | ||
<br><br> | |||
[2] Jose, J., J.E. Snyder, R.J. Kuhn. “A structural and functional perspective of alphavirus replication and assembly”. Future Microbiology 4 (2009): 837-856 | |||
<br><br> | <br><br> | ||
[3] Weaver, S.C., W.K. Reisen. “Present and future arboviral threats”. Journal of Antiviral Reseach 85 (2010): 328-345 | |||
<br><br> | |||
[4] Croddy, Eric C. and Hart, C. Perez-Armendariz J., <I>Chemical and Biological Warfare,</I> (Google Books), Springer, 2002, pp. 30-31, (ISBN 0387950761) | |||
<br><br> | |||
[5] CDC list of Bioterrorism Agents/Diseases http://www.bt.cdc.gov/agent/agentlist-category.asp | |||
<br><br> | |||
[6] Zacks, M.A., S., Paessler. “Encephalitic alphaviruses”. Journal of Veterinary Microbiology 140 (2010): 281-286 | |||
<br><br> | |||
[7] Slobodan, P., S.C., Weaver “Vaccines for Venezuelan equine encephalitis” Vaccine 27 (2009): D80-D85 | |||
<br><br> | |||
[8] Weaver, S.C., A.D.T., Barrett “Transmission cycles, host range, evolution and emergence of arboviral disease” Nature Reviews; Microbiology 2 (2004): 789-801 | |||
<br><br> | |||
Page authored for [http://biology.kenyon.edu/courses/biol375/biol375syl08.htm BIOL 375 Virology], September 2008 | Page authored for [http://biology.kenyon.edu/courses/biol375/biol375syl08.htm BIOL 375 Virology], September 2008 | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 06:48, 10 September 2010
Venezuelan equine encephalitis virus (VEEV)
A Viral Biorealm page on the family Venezuelan equine encephalitis virus
Baltimore Classification
Group IV: (+) sense single-stranded RNA viruses
Higher Order Categories
Family: Togaviridae
Genus: Alphavirus
Complex: New World
Description and Significance
Venezuelan equine encephalitis virus (VEEV) is an important animal and human pathogen found only in the Americas (the New World). VEEV is actually a complex of 7 different species as well as multiple subtypes and varieties [3]. Depending on the subtype, VEEV maintains either an enzootic or an epizootic life cycle. Enzootic species sustain a chain of transmission between a rodent reservoir and mosquito vector. Epizootic species cause epidemics in horses and other animals of agricultural importance. Most human cases of VEEV result from spill-over into the human population from an epizootic outbreak. Human are only incidental and usually dead-end hosts.
In the 1960's VEEV was weaponized by the U.S. and the U.S.S.R. [4]. The virus is considered a category B select bioterrorism threat by the U.S. government because it causes only moderate morbidity and low mortality in humans but severe morbidity and mortality in animals.
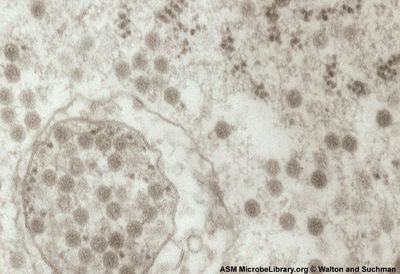
The virus has a biosafety level of 3 (BSL 3). It can only be worked with inside of specially equipped laboratories designed to deal with BSL 3 agents [5]. although VEEV is transmitted by mosquitoes in nature it is a very good aerosol. The virus has been implicated in more than 150 cases of accidental laboratory exposures that were not associated with cuts or needle pricks [6].
VEEV is an arbovirus (arthropod-virus), a general term for a virus that is transmitted via a hematophagous arthropod vector such as mosquitoes, flies and ticks. To be considered a true arbovirus there must be replication of the virus within the vector and VEEV is no exception [3].
The genus alphaviruses is included in the general group of Alphaviruses cause arthritic and encephalitic disease in mammals. This group includes the pathogens O'nyong'nyong virus, Chikungunya virus, Western equine encephalitis virus, Eastern equine encephalitis virus, and Venezuelan equine encephalitis. Alphaviruses are not only important human and animal pathogens but have also been used as model systems for the study of enveloped virus structure and as viral vectors in gene therapy research [2].
Genome Structure
VEEV has a non-segmented 11.4 kb (+) sense ssRNA genome with a poly (A) tail. The genome contains two reading frames that encode for two different polyproteins. Two-thirds of the genome starting from the 5’ end encode for a polyprotein that contains the four nonstructural proteins nsP1, nsP2, nsP3, and nsP4 (viral RNA-dependent RNA polymerase) that are required for genome replication. The last third of the genome encodes for a polyprotein with the capsid and envelope proteins CP, pE2, 6K, and E1 [7].
The viral RNA itself contains four conserved sequence elements (CSEs) that interact with various viral and host proteins involved in the replication, transcription, and packaging of viral RNA. A small non-translated region at the beginning of the 5’ end of the genome (CSE1) appears to fold up into a stem-loop structure that acts as a promoter for (+) RNA strand replication from a (-) RNA template. CSE2 is found near the 5’ end as well but it is within the coding region for nsP1 and may be a promoter for the synthesis of (-) strand template RNA from genomic (+) strand RNA. CSE4, located just upstream of the poly (A) tail near the 3’ end appears to be involved in promoting the synthesis of template RNA as well. CSE3 is located between the two ORFs and is required for transcription of subgenomic RNA (smaller sections of mRNA that encode for only part of the genome). Just upstream Near CSE4 there are also repeated sequence elements 40-60-nt in length that bind to host proteins involved in the translation of viral RNA [2].
Virion Structure of Venezuelan equine encephalitis virus
Venezuelan equine encephalitis virus has an enveloped isohedral nucleocapsid. The capsid has a triangulation of t=4. Virions appear round and are 65-70 nm in diameter. The envelope surrounding the nucleocapsid is derived from host lipid bilayer embedded with approximately 240 copies of E1 and E2 glycoproteins. The glycoproteins form heterodimer spikes that link up in an isohedral lattice that also displays t=4 symmetry like the Nucleocapsid [2].
Reproductive Cycle of Venezuelan equine encephalitis virus in a Host Cell
VEEV virions attach to a host receptor via the glycosylated envelope protein E2 and possibly E1. The exact host receptor for VEEV is unknown but it is probably fairly common because the virus can infect vertebrate (mammals) as well as invertebrate (mosquitoes) cells. One hypothesis is that single amino acid substitutions in the E2 protein allow the virus to attach to multiple cell surface receptors.
Once attached to the receptor the virus is endocytosed and the pH of the endosome drops and causes the E1-E2 dimer to destabilize and then trimerize with the late endosomal membrane. This trimerization allows for the fusion of the viral envelope with the endosome membrane and the eventual escape of the nucleocapsid into the cytoplasm. The exact mechanism of nucleocapsid uncoating in the cytoplasm is not known but may be related to some sort of destabilization by the initial drop in pH in the endosome.
(+) Genomic RNA is translated using host ribosomes and the nonstructural polyprotein is processed and the resulting proteins form replication complexes within vacuoles derived from lysosomal and endosomal membranes. Replication complexes begin synthesis of (-) template RNA from which both genomic RNA and subgenomic RNA are transcribed. The subgenomic RNA only encodes for the structural polyprotein that contains envelope and capsid components. The capsid subunit CP autoproteolyses and self assembles into nucleocapsids in the cytoplasm and genomic RNA is encapsidated by an unknown mechanism [2].
The remaining segment of polyprotein translocates into the ER. E1 and pE2 are folded and glycosylated in the ER and then taken to the golgi body where pE2 is cleaved to form E2 and E3. E3 seems to be involved with the dimerization between E1 and E2 but its exact role in viral replication has yet to be elucidated [2]. From the golgi the E1-E2 heterodimer is transported to the plasma membrane where E2 is required for nucleocapsid fusion with the plasma membrane and eventual viral budding.
During infection VEEV turns off cellular transcription and translation which leads to a decreased response from the innate immune system. Infection of the cell also initiates apoptosis by some unknown mechanism [2].
Viral Ecology & Pathology
In nature VEEV is only transmitted by mosquitoes but aerosol transmission has been observed in the laboratory. Mosquitoes become infected after feeding on infected blood. The virus eventually escapes the gut of the insect and makes its way to the salivary glands where it replicates. The new host is infected when virus laden saliva is injected into the skin during feeding [3].
VEEV can either sustain an enzootic or epizootic life cycle. Enzootic subtypes have a smaller host range and are transmitted between reservoir rodent hosts by mosquitoes of the genus Culex . These subtypes (II-IV) rarely cause disease unless a susceptible human or horse ends up in the middle of the transmission cycle. In order for an enzootic strain to start an epizootic There must be changes in genotype as well as geographic cross-over between susceptible animals and mosquito vectors. This can occur when there is a dramatic change in mosquito habitat such as an unusually wet season that allows the insects to move beyond their normal geographic range. Susceptible hosts must be within geographic range of the vector swamps and forests (this often times occurs when land is cleared for agricultural use) [3].
The epizootic subtypes of VEEV are IAC and IC. They are responsible for most major human and equine (horse) epidemics. Epizootic subtypes arise from changes in the amino acid sequence of the E2 envelop protein. Even single amino acid substitutions can result in epizootic strains. E2 protein is responsible for binding to host receptors so changes in the structure of this protein affect host range which in turn allows for zoonotic outbreaks (epidemics in animals) that can incidentally involve humans [8]. Changes in E2 structure allow mosquitoes of the genera Ochlerotatus and Psorphora to transmit the disease to horses and other mammals that display high titres of virus in their blood (viremia). This viremia is important in maintaining an epidemic. Luckily for the animals that survive infection (mortality is high) the antibodies produced by the immune systems are strong enough to prevent reinfection.
VEEV in humans presents mostly as a flu-like illness and is often times missed because of a lack of surveillance and laboratory diagnosis. Disease mortality is less than 1% and (encephalitic) only 14% of cases involve the central nervous system. In fatal human cases there is edema, congestion, hemorrhages, meningitis, and encephalitis of the brain and spinal cord. The lungs, liver and lymphoid tissue are also ravaged by the virus [8].
In horses, epizootic VEEV has very high mortality (50-90%). It causes swelling of the brain (encephalitis) that often-times result in fatal seizures [1].
Vaccines and Treatments
Currently treatment of VEEV infection is mostly supportive because there are no specific drugs for alphaviruses.
There is currently a vaccine available for both humans and horses. The live attenuated vaccine known as TC-83 is a strain of VEEV that was passed 83 times in guinea pig heart cells [7]. There is also an inactivated form of the vaccine known as C-84 derived from the TC-83 strain. Currently only the C-84 vaccine is licensed for use in horses in the U.S. although countries such as Mexico and Colombia still produce the live vaccine for horses. In the U.S. only at risk military and laboratory personal are vaccinated with the TC-83 strain and some receive C-84 boosters if initial vaccination did not produce sufficient immunity. The vaccine does have side effects that ranged from mild to moderate and did not provided full protection of nonhuman primates challenged by aerosol exposure the route of transmission most likely if VEEV were to be used in a biological terrorist attack [7].
References
[1] Walton, T., E., Suchman. “Venezuelan Equine Encephalitis (VEE) Virus” American Society for Microbiology. MicrobeLibrary.org. http://www.microbelibrary.org/asmonly/details.asp?id=2407
[2] Jose, J., J.E. Snyder, R.J. Kuhn. “A structural and functional perspective of alphavirus replication and assembly”. Future Microbiology 4 (2009): 837-856
[3] Weaver, S.C., W.K. Reisen. “Present and future arboviral threats”. Journal of Antiviral Reseach 85 (2010): 328-345
[4] Croddy, Eric C. and Hart, C. Perez-Armendariz J., Chemical and Biological Warfare, (Google Books), Springer, 2002, pp. 30-31, (ISBN 0387950761)
[5] CDC list of Bioterrorism Agents/Diseases http://www.bt.cdc.gov/agent/agentlist-category.asp
[6] Zacks, M.A., S., Paessler. “Encephalitic alphaviruses”. Journal of Veterinary Microbiology 140 (2010): 281-286
[7] Slobodan, P., S.C., Weaver “Vaccines for Venezuelan equine encephalitis” Vaccine 27 (2009): D80-D85
[8] Weaver, S.C., A.D.T., Barrett “Transmission cycles, host range, evolution and emergence of arboviral disease” Nature Reviews; Microbiology 2 (2004): 789-801
Page authored for BIOL 375 Virology, September 2008
