Vibrio cholerae pathogenesis
Vibrio cholerae is the causative agent of the diarrheal disease cholera. A disease affecting 2.8 million people per year and resulting in the deaths of 91,000, cholera is most common in areas with high population density and low sanitation quality.[1] [2] Not all V. cholerae are pathogenic: only two strains of serogroups O1 and O139 cause cholera.[1] Furthermore, it is considered a facultative human pathogen, as it primarily inhabits surface waters, as opposed to the small intestine.[3]
When entering and colonizing the human host, V. cholerae must endure changing environmental factors such as temperature, acidity, osmolarity, intestinal growth inhibitory substances, and immune system factors.[1] After sufficient colonization, an autoregulatory loop controlling ToxT, cholera toxin, and the toxin-coregulated pilus (TCP) results.[4] Understanding pathogenesis of V. cholerae requires the understanding of colonization and this loop.
Colonization of the Human Small Intestine
Biofilm Formation
V. cholerae’s journey through the acidic stomach is aided by its assembly into a biofilm, a structure consisting of bacteria in a matrix of sugars and proteins.[1] [3] 100% of V. cholerae cells in biofilms have been shown to withstand acid shock characteristic of the human stomach. Exposure to an environment with a pH of 4.5 for 15 to 30 minutes improves cell survival 50 and 1000-fold, respectively, compared to non-biofilm-associated cells.[5]
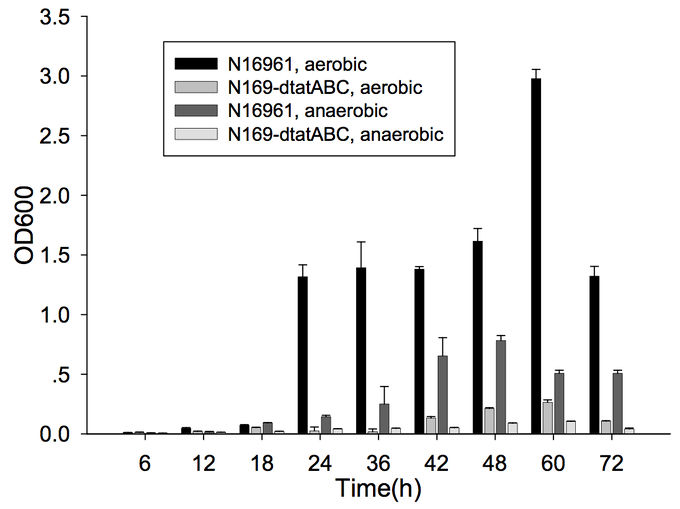
Furthermore, cells capable of forming biofilms have greater intestinal colonization capacity in the suckling mouse model. The rugose variant of V. cholerae, which has superior biofilm-forming abilities compared to the smooth variant, is also more successful at colonizing the intestine.[6] In addition, the twin-arginine translocation (Tat) system has also been shown to be important for biofilm formation and for colonization. The Tat system is an export system that recognizes the twin arginine signal peptide on proteins that are intended to be translocated across the cell membrane (these are often enzymes involved in the electron transport chain). This system has already been associated with virulence of Pseudomonas aeruginosa, Escherichia coli, Yersinia pseudotuberuclosis, and other pathogenic bacteria. The Tat system has recently been shown to be functional in V. cholerae; deletion of tatABC causes a decrease in biofilm formation (Figure 1) as well as in colonization of the suckling mouse.[7] Finally, the absence of vibrio polysaccharide (encoded by vps opeon), the major exopolysaccharide component of biofilms, or the absence of RbmA, one of the matrix proteins, leads to defective colonization.[6]
However, quorum sensing studies in V. cholerae have shown that the biofilm is not necessary during colonization, but instead during the bacteria’s travel through the acidic stomach, during which it needs a protective structure. Once they reach the intestine, they will then utilize virulence factors, not the biofilm, to colonize and infect the host.
Quorum Sensing
Toxin-Coregulating Pilus
Cholera Toxin
ToxR and ToxT Regulon
References
[1] Reidl, J. and K.E. Klose, Vibrio cholerae and cholera: out of the water and into the host. Fems Microbiology Reviews, 2002. 26: p. 125-139.
[2] Ali, M., et al., The global burden of cholera. Bulletin of the World Health Organization, 2012. 90: p. 209-218.
[3] Sack, D.A., et al., Cholera. Lancet, 2004. 363: p. 223-233.
[4] Yu, R.R., and V.J. DiRita, Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae. Journal of Bacteriology, 1999. 181: p. 2584-2592.
[5] Zhu, J. and J.J. Mekalanos, Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Developmental Cell, 2003. 5: p. 647-656.
[6] Fong, J.C.N., et al., Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology, 2010. 156: p. 2757-2769.
[7] Zhang, L., et al., Pleiotropic effects of the twin-arginine translocation system on biofilm formation, colonization, and virulence in Vibrio cholerae. Biomed Central Microbiology, 2009. 9: p. 1-13.
Edited by Tina Solvik, a student of Suzanne Kern in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2015.
