Virulence of Pseudomonas savastanoi
Hildy Joseph, Kenyon 2013
Introduction

Olive knot disease, which is characterized by knots and galls on emerging stems, branches, and occasionally leaves, was first described in the fourth century B.C. by the Greek philosopher Teophrastus.

Pseudomonas savastanoi is the bacteria responsible for olive knot disease (Iacobellis 2001). Infection occurs through wounds induced by pruning or severe weather occurrences, including freezing and hail (Young 2004). Plants experience death and decay, including reduced limb size and plant size as well as defoliation, within eight months of infection (Iacobellis 2001). The overall vigor of olive trees inoculated with P. savastanoihas been shown to decline (Quesada et al. 2009). Knots tend to form in the spring during the most active growth period (Young 2004). Plant vulnerability is also increased in the autumn, in part because of other pathogens like the fungus Spilocaea oleaginea target olive trees at the same time. Thus, the chance of co-infection by
P. savastanoiis heightened (Teviotdale and Krueger 2004). The bacterium spreads easily within an orchard via wind or pruning tools (Young 2004). Moreover, once infected, P. savastanoi can spread to new wound sites within the same plant (Penyalver et al. 2006). Olive quality from infected plants is greatly reduced. Olives from infected plants are bitter, salty, sour, or rancid (Iacobellis 2001). Yield is also negatively impacted by the disease. Olive knot disease is a global issue and has been found in parts of Europe, Asia, Africa, North America, South America, and Australia. The incidence and degree of virulence varies by location (Young 2004).
Understanding the virulence of P. savastanoi is important because of the emerging economic significance of olives and olive oil.

Olive oil comprises a major part of the Mediterranean diet, which is becoming increasingly popular because of its health benefits. The Mediterranean diet has been associated with a plethora of health benefits, reducing LDL-cholesterol and improving HDL-cholesterol, which lessens the risk for heart disease, as well as improvement in digestion and autoimmune or inflammatory diseases (Bertolini et al. 2003 and Alarcon de la Lastra et al. 2001). Furthermore, crops are often grown as monocultures. Reduced genetic diversity can increases susceptibility to disease. Thus, understanding plant pathogens is essential for preserving essential food sources (Grinter et al. 2012).
Classification of P. savastanoi
The genus Psuedomonas consists of Gram-negative bacteria that are highly prevalent in nature and includes phytopathogenic as well as nonpathogenic species (Janse 1982 and Yamamoto et al. 2000). P. savastonoi is a nonsporeforming bacteria that contains several flagella for motility (Janse 1982). The genus also includes the human pathogen Pseudomonas aeruginosa (Hancock et al. 1998). The occurrence of phytopathogeny is polphyletic among species of Pseudomonas, which provides evidence for horizontal gene transfer. The virulence genes may have first arisen in the common ancestor for the clade of plant pathogens within Pseudomonas, termed the 'P. syringae complex', which includes P. savastanoi. More recently evolutionarily, the virulence genes may have been transferred horizontally to other species in Pseudomonas. The complex comprises a pathogenicity island. (Yamamoto et al. 2000). Generally, pathogens in the P. syringae complex cause leaf death and cankers, but P. savastanoi pv. savastanoi has a unique pathogenicity for the complex (Ramos et al. 2012).
P. savastonoi was originally thought to be one of forty-five pathovars of Pseudomonas syringae due to similar biochemistry and physiology. DNA-DNA hybridization data revealed that Pseudomonas savastanoi had sufficient genetic distinction from P. syringae to merit its own species classification. Several pathovars were subsequently attributed to P. savastanoi, including P. savastanoi pv. savastanoi, P. savastanoi pv. glycinea, and P. savastanoi pv. phaseolicola (Gardan et al. 1992). Other pathovars have subsequently been recognized, including P. savastanoi pv. fanxini and P. savastanoi pv. nerii.
Host range and susceptibility
Cultivated and wild olives (Olea europaea) and ash (Fraxinus excelsior) are susceptible to tumor induction by P. savastanoipv. savastanoi. Other pathovars have different host ranges. For example, P. savastanoipv. nerii infects oleander (Nerium oleander) but can also infect olives. P. savastanoi pv. savastanoi has been shown to infect oleander, but the occurrence is unusual (Ramos et al. 2012). Though P. savastanoi pv. savastanoi is generally limited to olive and oleander hosts, there is a recent report of a
P. savastanoiinfection on Mandevilla sanderi, commonly known as Brazilian jasmine (Eltlbany et al. 2012). There are several other pathogenic pathovars. P. savastanoi pv. glycinea infects soybeans and P. savastanoi pv. phaseolicola is responsible for halo blight in beans (Gardan et al. 1992).
Resistant olive cultivars are rare, though susceptibility to P. savastanoidoes vary. Young olive plants are particularly non-resistant to infection (Penyalver et al. 2006). P. savastanoican be epiphytic upon infection (Rodriguez-Moreno et al. 2009).
Mechanisms of Virulence
Plant hormones: cytokinin and IAA
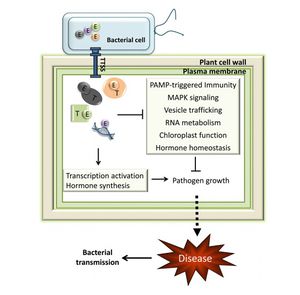
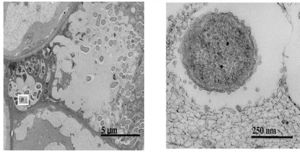
Infection by Pseudomonas savastanoi causes cavities in host cells in which bacteria can aggregate (Marchi et al. 2009). P. savastanoi also accumulate in the xylem, which suggests a mechanism, heretofore unverified, of bacterial transport throughout the plant (Rodriguez-Moreno et al. 2009).
Plant hormones are one aspect of virulence that activates tumorigenesis. Cytokinin and IAA production both contribute to knot formation by interfering with endogenous signals to induce rapid proliferation of host cells at the site of infection (Surico et al. 1985, Marchi et al. 2009, and Sisto et al. 2004). Pseudomonas savastanoi carry plasmids that encode for indole-3-acetic acid (IAA), a protein involved in auxin signaling (Surico et al. 1985 and Muto et al. 2007). Various genetic studies confirm the importance of IAA and cytokinins for virulence. Mutants lacking either IAA or cytokinins were less symptomatic upon infection of a host olive plant. Infections were limited to some of the normal occurrences, such as leaf necrosis and stem swelling. Tumor induction can be induced by only IAA, though cytokinins also play an important role (Iacobellis 1994). Strains lacking the pIAA1 plasmid, which encodes for the two genes responsible for IAA production, iaaM and iaaH, fail to induce olive knot growth in hosts plants (Spaepen and Vanderleyden 2010). Auxin may be important for virulence by downregulating factors that contribute to the host defense. For example, auxin can protect against the hypersensitive response, a defense mchanism in plants injected with P. syringae (Melotto and Kunkel 2013). HR is a form of programmed cell death in host cells and is widely conserved among plants and animals (Grant et al. 2006). Auxin has many additional roles. It may regulate gene expression or affect the physiology of host cells, for example (Melotto and Kunkel 2013).
P. savastanoi occur in conjunction with other bacterial species within olive knots. It is hypothesized that phytohormones produced by the nonpathogenic bacteria coexisting in olive knots may influence P. savastanoi (Ramos et al. 2012). Auxin is produced by a variety of other genera and species that are common to olive knots, including Stenotrophomonas maltophila, Pantoea agglomerans, Pantoea oleae, Burkholderia cepacia, and Hafrnia alvei, all of which are other Gram-negative rod forming bacteria. Furthermore, IAA specifically is produced by other Pseudomonas species (Ouzari et al. 2008).
Suppressing host immune response and defense mechanisms
The P. Syringae complex, composed of the hrp/hrc gene cluster, acts on host the immune system (Grant et al. 2006 and Sisto et al. 2004). Defense mechanisms in plants involve mechanical resistance to pathogens through the cell wall. Additionally, plants contain a set of receptors that respond to microbe-associated molecular patterns and induce the production of antimicrobial agents (Grant et al. 2006). The hrp genes encode for a type III protein secretion system (T3SS). The T3SS is comprised of a pilus that connects the bacterium with the host membrane and the pore that forms in the host cell to allow entry of type III effector proteins (Grant et al. 2006). T3SS are found in Gram-negative bacteria that interact eukaryotes, both as pathogens or symbionts, such as nitrogen-fixing Rhizobium (Gazi et al. 2012). The complex can overcome plant cell walls with the aid of helper proteins that are secreted into the extracellular space (Grant et al. 2006). Host cell wall degradation has been observed in in olive knots (Rodriguez-Moreno et al. 2009). All of the proteins associated with the T3SS are encoded for in the P. Syringae pathogenicity island (Grant et al. 2006).
The T3SS proteins evolved from a common ancestor and the genomic island encoding all of the associated proteins was transferred horizontally among seven families. P. savastanoi and other Pseudomonads exhibit the Hrc-Hrp2-T3SS family. The other genera that share T3SS-2 are Erwinia, Ralstonia, and Xanthomonas (Gazi et al. 2012).
P. savastanoi contain the hrp/hrc gene cluster that encodes for the TTSS and related proteins (Grant et al. 2006). One emerging area of research is understanding the specific effectors and other elements of the T3SS (Rodriguez-Palenzuela et al. 2010). The gene clusters are essential to P. savastanoi virulence and may be more important pathologically than auxin and cytokinin action. The hrp gene cluster reduces plant vitality by inducing leakage of essential nutrients into the extracellular milieu, in addition to its roles inhibiting the plant immune system. The closely related hrc genes are responsible for the plant HR (Sisto et al. 2004). HR is a form of programmed cell death in host cells and is widely conserved among plants and animals (Grant et al. 2006).
In addition to the T3SS, other interactions with host defense mechanisms have been postulated. Plants produce a range of secondary metabolites that aid in defense. Flavonoids are one class of phenolic compounds that are protective against parasites and pathogens (Treutter 2005). Flavonoids, along with other phenolic compounds like secoiridoids and lignans are common to olive oil and confer some of the major health benefits associated with the Mediterranean diet (Christophoridou et al. 2005 and Alarcon de la Lastra et al. 2001). Sequence data and empirical evidence in P. savastanoi reveals the potential for phenol catabolism. Other pathogens of woody plants, in which phenolic defense mechanisms are common, show similar catabolic abilities (Rodriguez-Palenzuela 2010). Furthermore, olive knots induced by P. savastanoi pv. savastanoi show a higher presence of phenolic compounds than in other leafs and shoots (Cayuela 2005). Thus, P. savastanoi may circumvent the the secondary metabolites produced by plants to preempt and actively combat bacterial infections by catabolizing the compounds (Rodriguez-Palenzuela 2010).
Emerging views of virulence: signaling mechanisms and outer membrane vesicles
The P. savastanoi signaling mechanism is another quality that has been implicated in its virulence. Gram-negative bacteria commonly use N-acyl homoserine lactone (AHL) signaling molecules. P. savastanoi commonly occurs in association with P. agglomerans and E. toletana, which are both nonpathogenic Enterobacteriaceae that frequently grow at olive knot infection sites (Hosni et al. 2011). In general, plants that have been inoculated with P. savastanoi have a higher overall bacteria population than plants that do not bear an infection by P. savastanoi (Quesada et al. 2009). AHL signals can be shared across P. agglomerans, E. toletana, and P. savastanoi. Specifically, the association of E. toletana and P. savastanoi confers synergistic pathogenic effects. The interactions between E. toletana and P. savastanoi are stable and lead to an incidence of greater olive knot formation (Hosni et al. 2011). Alternatively, interactions between P. savastanoi and resident microbial communities on host plants may negatively impact bacterial virulence (Hosni et al. 2011 and Dulla and Lindow 2009). The pathogenicity of P. Syringae pv. syringae, which is closely related species to P. savastanoi pathovars, was reduced by crosstalk with signaling molecules from the resident bacterial populations. Instead of synergistic effects, the AHLs from other species interfered with AHL signaling in P. Syringae (Dulla and Lindow 2009). Researchers are working on exploiting the interactions between resident populations and P. savastanoi to reduce its incidence and virulence (Krid et al. 2011).
Additionally, P. savastanoi employ a unique mechanism for plant pathogens by inducing the release of outer membrane vesicles form the host cell. Animal pathogens commonly release outer membrane vesicles for transporting virulence factors into host cells. Their role in P. savastanoi infection may be a relating to correcting for osmotic pressure (Perez-Martinez et al. 2010).
Development of Genetic Tools for Understanding Virulence
Major advances in the last several years have rapidly progressed the understanding of P. savastanoi after decades of stalled research. Before 2007, the significant impediment to studying P. savastanoi was the lack of any strains that were amenable to genetic manipulation, specifically conjugation. The problem is consistent across other Pseudomonads that infect woody plants, including P. savastanoi pvs. Savastanoi, franxini, and nerii. Several strains were isolated that are have more efficient transformations (Perez-Martinez 2007). One of the strains with the highest transformation efficiency, P. savastanoi pv. savastanoi NCPPB 3335, was recently sequenced (Perez-Martinez 2007 and Rodriguez-Palenzuela et al. 2010). A draft genome was obtained with pyrosequencing and compared to other published P. Syringae genomes. The primary goal of the genetic manipulation is to augment the current poor understanding of P. savastanoi virulence (Rodriguez-Palenzuela et al. 2010).
The draft genome of P. savastanoi pv. savastanoi NCPPB 3335 revealed 73 genes that are not found in other species as well as eight gene inversions in comparison with closely related species. Researchers suggest that the basis for host specificity is encoded for in the 73 genes unique to P. savastanoi (Silby et al. 2011 and Rodriguez-Palenzuela et al. 2010).
Other emerging techniques have recently allowed for easier plant manipulation and greater resolution. For example, cultivation by micropropagation circumvents the arduous growing requirements for olive trees, including long growing seasons and large plant size. Additionally, the draft P. savastanoi pv. savastanoi NCPPB 3335 genome has been employed to develop a green fluorescent protein tagging system. Scanning and transmission electron microscopy can be used to visualize the bacteria in planta and further elucidate virulence mechanisms (Ramos et al.2012).
Responding to Olive Knot Disease in Crop Management
Detection of P. Savastanoi
An infection can be recognized on crop plants in the late stages of infections. Insect wounds are easily confused with olive knots in the preliminary onset. Bacteria can be isolated from knots and cultured in the laboratory for identification, though older knots generally contain secondary invaders (Young 2004). However, plants can be infected without having visible symptoms (Bertolini et al. 2003b). Furthermore, the bacteria grows relatively slowly in the laboratory, with a required incubation between two to three days at 27°C (Janse 1982 and Young 2004). Other techniques have been developed to rapidly detect P. savastanoi without culturing or the necessity of serological methods. For example, there is a highly sensitive PCR technique based on amplification of the iaaL gene, which encodes for a product that catalyzes the addition of lysine to IAA (Bertolini et al. 2003b). Isolates can be distinguished by sequence differences, specifically differing numbers of nucleotide motif tandem repeats, in both iaaL paralogs found in P. savastanoi strains (Perez-Martinez 2008). Another technique using rt-PCR can simultaneously detect P. savastanoi along with four RNA viruses. Detecting multiple plant pathogens in a single reaction conserves time and resources (Bertolini et al. 2003a).
Control of the pathogen
Farmers have a variety of options to alleviate the problem of olive knot disease, though noncommercial farmers have fewer available products. Landscapers and homeowners are advised to practice several strategic planting practices to minimize the chances for disease dissemination. For example, timing of olive tree purchase is critical, as P. savastanoi is often epiphytic during the winter months and infections are most visible in late spring. Furthermore, visible galls should be removed with sterile pruning tools to minimize spread of the bacteria (Fichtner 2011). The levels of epiphytic bacteria may be positively correlated with the occurrence of olive knots, and thus minimizing the presence of olive knots reduces the overall spread of the infection (Quesada et al. 2010). One measure to preemptively combat disease is to remove oleander plants that are growing in close proximity to growing area because of the potential overlap in host specificity for P. savastanoi (Fichtner 2011). However, P. savastanoi is generally an obligate symbiont and does not proliferate on soil (Ramos et al. 2012).
Resistant olive cultivars would be the best method for controlling P. savastanoi. However, naturally resistant cultivars have not yet been detected. Recently, susceptibility measures to P. savastanoi have been standardized, which will aid in developing cultivars that will not become infected (Ramos et al. 2012).
Chemical
Copper compounds are commonly used to reduce P. savastanoi proliferation (Krid et al. 2011). The Cu2+ ions can be toxic to P. savastanoi. Copper can also induce a viable but nonculturable state, which interferes with serological detection of P. savastanoi on host plants (Quesada et al. 2010). However, the method poses threats to the environment and public health and also has the disadvantage of reduced efficacy with continued use (Krid et al. 2011). Several copper compounds are available, including copper oxychloride and cuprocalcic suflate plus mancozeb. Both compounds reduced the amount of P. savastanoi growing on the leafs and stems (Quesada et al. 2010). Maximal efficacy of treatment requires a minimum of biannual application in the spring and fall, when olive trees are at the highest risk for infection. Olive trees are predisposed for infection when leafs fall in the spring due to the residual leaf scarring as well as during frosts in the late autumn (Teviotdale and Krueger 2004). Bacteria that resist the copper treatment and exist in epiphytic and endophytic states can proliferate with failure to adhere to a regular copper treatment schedule (Quesada et al. 2010). Farmers are recalcitrant toward biannual copper application because of its effect on the olive product (Teviotdale and Krueger 2004).
Polyphenols have also demonstrated efficacy in controlling olive knot. The compounds were obtained in extracts of olive mill waste water. Even at the lowest concentration tested, 100 mg/L, all olive knot formation was blocked, whereas a concurrent copper treatment was less effective (Krid et al. 2011).
Biological
Other bacterial species can be used to control the incidence of P. savastanoi. Antimicrobial proteins produced by other microbes include colicin-like bacteriocins. Bacteriocins are common bacterial protein products that are detrimental to closely related species. Bacteriocins from P. Syringae pv. ciccaronei can inhibit both olive knot growth as well as epiphytic P. savastoni infection (Grinter et al. 2012). Another promising development in the biological control of olive blot is the application of Bacillus subtilis. B. subtilis produces antibiotics like zwittermicin-A and kanosamine as well as other antimicrobial lipoproteins. A protein produced from B. subtilis has been shown to reduce the size of olive knots caused by P. savastanoi (Krid et al. 2012).
References
Alarcon de la Lastra, C., M.D. Barranco, V. Motilva, J.M. Herrerias. 2001. Mediterranean diet and health: biological importance of olive oil. Current Pharmaceutical Design 7(10):933-950.
Bertolini, E., A. Olmos, M.M. Lopez, M. Cambra. 2003a. Multiplex nested reverse transcription-polymerase chain reaction in a single tube for sensitive and simultaneous detection of four RNA viruses and Psuedomonas savastanoi pv. savastanoi in olive trees. Phytopathology 93(3):286-292.
Bertolini, E., R. Penyalver, A. Garcia, A. Olmos, J.M. Quesada, M. Cambra, M.M. Lopez. 2003b. Highly sensitive detection of Pseudomonas savastanoi pv. savastanoi in asymptomatic olive plants by nested-PCR in a single closed tube. Journal of Microbiological Methods 52(2):261-266.
Cayuela, J.A., M. Rada, J.J. Rios, T. Albi, A. Guinda. 2006. Changes in phenolic composition induced by Pseudomonas savastanoi pv. Savastanoi infection in olive tree: presence of large amounts of verbascoside in nodules of tuberculosis disease. Journal of Agricultural and Food Chemistry 54(15):5363-5368.
Christophoridou, S., P. Dais, L.H. Tseng, M. Spraul. 2005. Separation and identification of phenolic compounds in olive oil by coupling high-performance liquid chromatography with postcolumn solid-phase extraction to nuclear magnetic resonance spectroscopy (LC-SPE-NMR). Journal of Agricultural and Food Chemistry 53(12):4667-4679.
Dulla, G.F. and S.E. Lindow. 2009. Acyl-homoserine lactone-mediated cross talk among epiphytic bacteria modules behavior of Pseudomonas syringae on leaves. Multidisciplinary Journal of Microbial Ecology 3:825-834.
Eltlbany, N., Z.Z. Prokscha, M.P. Castaneda-Ojeda, E. Krogerrecklenfort, H. Heuer, W. Wohanka, C. Ramos, K. Smalla. 2012. A new bacterial disease on Mandevilla sanderi, caused by Pseudmonas savastanoi: lessons learned for bacterial diversity studies. Applied Environmental Microbiology 78(23):8492-8297.
Gazi, A.D., P.F. Sarris, V.E. Fadouloglou, S.N. Charova, N. Mathioudakis, N.J. Panopoulos, M. Kokkinidis. 2012. Phylogenetic analysis of a gene cluster encoding an additional, rhizobial-like type III secretion system that is narrowly distributed among Pseudomonas syringae strains. BMC Microbiology 12:188.
Hosni, T., C. Moretti, G. Devescovi, Z.R. Suarez-Moreno, M.B. Fatmi, C. Guarnaccia, S. Pongor, A. Onofri, R. Buonaurio, V. Venturi. 2011. Sharing of quorum-sensing signals and role of interspecies communities in a bacterial plant disease. Multidisciplinary Journal of Microbial Ecology 5:1857-1870.
Iacobellis NS. 2001. Olive knot. In: Maloy OC, Murray TD, eds. Encyclopedia of plant pathology. New York, NY, USA: John Wiley & Sons, 7143–715.
Iacobellis, N.S., A. Sisto, G. Sucrico, A. Evidente, E. DiMaio. 1994. Pathogenicity of Pseudomonas syringae subsp. savastanoi mutants defective in phytohormone production. Journal of Phytopathology 140(3):238-248.
Janse, J.D. 1982. Pseudomonas syringae subsp. Savastanoi (ex Smith) subsp. Nov., nom. Rev., the bacterium casuing excrescences on Oleaceae and Merium oleander L. International Journal of Systemic and Evolutionary Microbiology 32(2):166-169.
Krid, S., M. Bouaziz, M.A. Triki, A. Gargouri, A. Rhouma. 2011. Inhibition of olive knot disease by polyphenols extracted from olive mill waste water. Journal of Plant Pathology 93(3):561.
Krid, S., M.A. Triki, A. Gargouri, A. Rhouma. 2012. Biocontrol of olive knot disease by Bacillus subtilis isolated from olive leaves. Annals of Microbiology 62(1):149-154.
Fichtner, E.J. 2011. Olive Knot: Integrated Pest Management for Home Gardeners and Landscape Professionals. University of California Statewide Integrated Pest Management Program Agriculture and Natural Resources. Publication 74156.
Gardan, L., C. Bollet, M. Abu Ghorrah, F. Grimont, P.A.D. Grimont. 1992. DNA relatedness among the pathovar strains of Pseudomonas syringae subsp. Savastanoi Janse (1982) and proposal of Psuedomonas savastanoi sp. nov. International Journal of Systemic and Evolutionary Microbiology 42(4):606-612.
Grant, S.R., E.J. Fisher, J.H. Chang, B.M. Mole, J.L. Dangl. 2006. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annual Reviews of Microbiology 60:425-449.
Grinter, R., J. Milner, D. Walker. 2012. Bacteriocins active against plant pathogenic bacteria. Biochemical Society Transactions 40(6):1498-1502.
Hancock, R.E.W. 1998. Resistance mechansims in Psuedomonas aeruginosa and other nonfermentative gram-negative bacteria. Clinical Infectious Diseases 27(S1):S93-S99.
Marchi, G., B. Mori, P. Pollacci, M. Mencuccini, G. Surico. 2009. Systemic spread of Pseudomonas savastanoi pv. Savastanoi in olive explants. Plant Pathology 58:152-158.
Melotto, M., B.N. Kunkel. 2013. Virulence strategies of plant pathogenic bacteria. In: The Prokaryotes – Prokaryotic Physiology and Biochemistry, eds. E. Rosenberg et al. Springer-Verlag, Berlin, pp. 61-82.
Muto, H., M.K. Watahiki, K.T. Yamamoto. 2007. What makes each Aux/IAA gene unique in its gene family, expression pattern or properties of the gene product? Plant Signaling and Behavior. 2(5):390-392.
Ouzari, H., A. Khsairi, N. Raddadi, L. Jaoua, A. Hassen, M. Zarrouck, D. Daffonchio, A. Boudabous. 2008. Diversity of auxin-producing bacteria associated to Pseudomonas savastanoi-induced olive knots. Journal of Basic Microbiology 48:370-377.
Penyalver, R., A. Garcia, A. Ferrer, E. Bertolini, J.M. Quesada, C.I. Salcedo, J. Piquer, J. Perez-Panades, E.A. Carbonell, C. del Rio, J.M. Caballero, M.M. Lopez. 2006. Factors affecting Pseudomonas savastanoi pv. savastanoi plant inoculaitons and their use for evaluation of olive cultivar susceptibility. Phytopathology 96(3):313-319.
Perez-Martinez, I., L. Rodriguez-Moreno, L. Lambertsen, I.M. Matas, J. Murillo, S. Tegli, A.J. Jimenez, C. Ramos. 2010. Fate of a Pseudomonas savastanoi pv. savastanoi type III secretion system mutant in olive plants (Olea europea L.). Applied and Environmental Microbiology 76(11):3611-3619.
Perez-Martinez, I., L. Rodriguez-Moreno, I.M. Matas, C. Ramos. 2007. Strain selection and improvement of gene transfer for genetic manipulation of Pseudomonas savastanoi isolated from olive knots. Research in Microbiology 158(1):60-69.
Perez-Martinez, I., Y. Zhao, J. Murillo, G.W. Sundin, C. Ramos. 2008. Global genomic analysis of Pseudomonas savastanoi pv. savastanoi plasmids. Journal of Bacteriology 190(2):625-635.
Quesada, J.M, R. Penyalver, J. Perez-Panades, C.I. Salcedo, E.A. Carbonell, M.M. Lopez. 2010. Comparison of chemical treatments for reducing epiphytic Pseudomonas savastanoi pv. Savastanoi populations and for improving subsequent control of olive knot disease. Crop Protection 29(12):1413-1420.
Quesada, J.M., R. Penyalver, J. Perez-Panades, C.I. Salcedo, E.A. Carbonell, M.M. Lopez. 2009. Dissemination in Pseudomonas savastanoi pv. savastanoi populations and subsequent appearance of olive knot disease. Plant Pathology 59(2):262-269.
Ramos, C., I.M Matas, L. Bardaji, I.M. Argon, J. Murillo. Pseudomonas savastanoi pv. savastanoi: some like it knot. Molecular Plant Pathology 13(9):998-1009. Rodriguez-Moreno, L., A.J. Jimenez, C. Ramos. 2009. Endopathogenic lifestyle of Pseudomonas savastanoi pv. Savastanoi in olive knots. Microbial Biotechnology 2(4):476-488.
Rodriguez-Palenzuela, P., I.M. Matas, J. Murillo, E. Lopez-Solanilla, L. Bardaji, I. Perez-Martinez, M.E. Rodriguez-Moskera, R. Penyalver, M.M. Lopez, J.M.
Quesada, B.S. Biehl, N.T. Perna, J.D. Glasner, E.L. Cabot, E. Neeno-Eckwall, C. Ramos. 2010. Annotation and overview of the Psuedomonas savastanoi pv. savastanoi NCPPB 3334 draft genome reveals the virulence gene complement of a tumour-inducing pathogen of woody hosts. Environmental Microbiology 12(6):1604-1620.
Silby, M.W., C. Winstanley, S.A.C. Godfrey, S.B. Levy, R.W. Jackson. 2011. Pseudomas genomes: diverse and adaptable. FEMS Microbiology Reviews 35(4):652-680.
Sisto, A., M.G. Cipriani, M. Morea. 2004. Knot formation caused by Pseudomonas syringae subsp. savastanoi on olives is hrp-dependent. Phytopathology 94(5):484-489.
Spaepen, S., J. Vanderleyden. 2010. Auxin and plant-microbe interactions. Cold Spring Harbor Perspectives in Biology 3(4):a001438.
Surico, G., N.S. Iacobellis, A. Sisto. 1985. Studieso n the role of indole-3-acetic acid and cytokinins in the formation of knots on olive and oleander plants by Pseudomonas syringae pv. Savastanoi. Physiological Plant Pathology 26:309-320.
Teviotdale, B.L., W.H. Krueger. 2004. Effects of copper sprays, defoliation, rainfall, and inoculum concentration on incidence of olive knot disease. Plant Disease 88:131-135.
Treutter, D. 2005. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biology 7:581-591.
Yamamoto, S., H. Kasai, D.L. Arnold, R.W. Jackson, A. Vivian, S. Harayama. 2000. Phylogeny of the genus Psuedomonas: intrageneric strucure reconstructed from the nucleotide sequences of gryB and rpoD genes.
Young, J.M. 2004. Olive knot and its pathogens. Australian Plant Pathology 33:33-39.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.