West Nile Virus in Birds: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
[https://microbewiki.kenyon.edu/index.php/West_Nile_Virus: West Nile Virus], of the family Flaviviridae, is a zoonotic disease that infects many species, including humans, throughout the world. Birds are the virus’s primary [http://en.wikipedia.org/wiki/Natural_reservoir: natural reservoir], where they act as the [http://en.wikipedia.org/wiki/Host_%28biology%29#Definitions: amplifier host]. but their host competency (their ability to perpetuate the virus' development and spread) and response to infection varies between species; they can have diverging [http://en.wikipedia.org/wiki/Viral_load: viral loads], [http://en.wikipedia.org/wiki/Viremia: viremia], [http://en.wikipedia.org/wiki/Viral_shedding: viral shedding], clinical signs, and morbidity. Many factors may affect a particular organism and/or species’ susceptibility and response to infection, based on their life histories, immune-response capabilities, previous immunities, breeding systems, genetics, and stress levels. Birds vary widely in how they are affected by West Nile Virus. | [https://microbewiki.kenyon.edu/index.php/West_Nile_Virus: West Nile Virus], of the family Flaviviridae, is a zoonotic disease that infects many species, including humans, throughout the world. Birds are the virus’s primary [http://en.wikipedia.org/wiki/Natural_reservoir: natural reservoir], where they act as the [http://en.wikipedia.org/wiki/Host_%28biology%29#Definitions: amplifier host]. but their host competency (their ability to perpetuate the virus' development and spread) and response to infection varies between species; they can have diverging [http://en.wikipedia.org/wiki/Viral_load: viral loads], [http://en.wikipedia.org/wiki/Viremia: viremia], [http://en.wikipedia.org/wiki/Viral_shedding: viral shedding], clinical signs, and morbidity. Many factors may affect a particular organism and/or species’ susceptibility and response to infection, based on their life histories, immune-response capabilities, previous immunities, breeding systems, genetics, and stress levels. Birds vary widely in how they are affected by West Nile Virus. | ||
<br> <br> | <br> <br> | ||
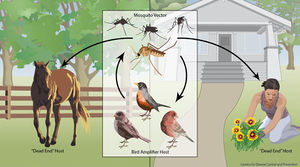
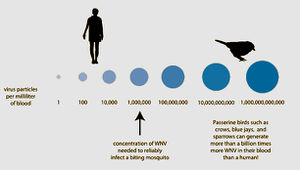
Most mammals, including horses and humans, are “dead-end” hosts for West Nile Virus, as, after infection, the virus does not become in high enough concentration in the blood to then be spread to insect vectors. Many bird species, however, are important “amplification-hosts” for the spread of West Nile Virus, as the virus multiplies to exorbitant numbers within them. Some species can generate the highest levels of virus particles in their blood presently known for any viral infection; they can have millions of times more viral particles in their blood than similarly infected mammals. | Most mammals, including horses and humans, are “dead-end” hosts for West Nile Virus, as, after infection, the virus does not become in high enough concentration in the blood to then be spread to insect vectors. Many bird species, however, are important “amplification-hosts” for the spread of West Nile Virus, as the virus multiplies to exorbitant numbers within them. Some species can generate the highest levels of virus particles in their blood presently known for any viral infection; they can have millions of times more viral particles in their blood than similarly infected mammals. [2 for now] | ||
==Initial Infection== | ==Initial Infection== | ||
| Line 125: | Line 125: | ||
==References== | ==References== | ||
<br> | <br> | ||
| Line 131: | Line 131: | ||
1. Komar, N., S. Langevin, S. Hinten, N. Nemeth, E. Edwards, D. Hettler, B. Davis, R. Bowen, and M. Bunning. 2003. [http://wwwnc.cdc.gov/eid/article/9/3/02-0628_article.htm Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus.] Emerging Infectious Diseases 9(3): 311-322. | 1. Komar, N., S. Langevin, S. Hinten, N. Nemeth, E. Edwards, D. Hettler, B. Davis, R. Bowen, and M. Bunning. 2003. [http://wwwnc.cdc.gov/eid/article/9/3/02-0628_article.htm Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus.] Emerging Infectious Diseases 9(3): 311-322. | ||
<br> | |||
2. Greene, S. E., and A. Reid. (2013). [http://academy.asm.org/index.php/faq-series/793-faq-west-nile-virus-july-2013 FAQ: West Nile Virus], July 2013. American Society For Microbiology. | |||
<br> | <br> | ||
Revision as of 04:11, 10 March 2014
West Nile Virus, of the family Flaviviridae, is a zoonotic disease that infects many species, including humans, throughout the world. Birds are the virus’s primary natural reservoir, where they act as the amplifier host. but their host competency (their ability to perpetuate the virus' development and spread) and response to infection varies between species; they can have diverging viral loads, viremia, viral shedding, clinical signs, and morbidity. Many factors may affect a particular organism and/or species’ susceptibility and response to infection, based on their life histories, immune-response capabilities, previous immunities, breeding systems, genetics, and stress levels. Birds vary widely in how they are affected by West Nile Virus.
Most mammals, including horses and humans, are “dead-end” hosts for West Nile Virus, as, after infection, the virus does not become in high enough concentration in the blood to then be spread to insect vectors. Many bird species, however, are important “amplification-hosts” for the spread of West Nile Virus, as the virus multiplies to exorbitant numbers within them. Some species can generate the highest levels of virus particles in their blood presently known for any viral infection; they can have millions of times more viral particles in their blood than similarly infected mammals. [2 for now]
Initial Infection
Birds may become infected with West Nile Virus in multiple ways. The most common occurrence is through a mosquito vector. However, studies have shown that they may contract the infection through eating infected organisms, direct contact, or fecal contact.
Subsection: Mosquito Vectors: Blah blah mosquitos usually something something Oral Transmission: By ingesting infected mice or other birds, a carnivorous bird may present viremia.
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Overall paper length should be 3,000 words, with at least 3 figures with data.
Species Susceptibilities and Competence
| Table 1. West Nile Virus Host Competency of 25 Species of Birds [1 for now]. | |
| Species | Reservoir Competence Index* |
|---|---|
| Blue Jay | 2.55 |
| Common Grackle | 2.04 |
| House Finch | 1.76 |
| American Crow | 1.62 |
| House Sparrow | 1.59 |
| Ring-billed Gull | 1.26 |
| Black-billed Magpie | 1.08 |
| American Robin | 1.08 |
| Red-winged Blackbird | 0.99 |
| American Kestrel | 0.93 |
| Great Horned Owl | 0.88 |
| Killdeer | 0.87 |
| Fish Crow | 0.73 |
| Mallard | 0.48 |
| European Starling | 0.22 |
| Mourning Dove | 0.19 |
| Northern Flicker | 0.06 |
| Canada Goose | 0.03 |
| Rock Dove | 0 |
| American Coot | 0 |
| Japanese Quail | 0 |
| Northern Bobwhite | 0 |
| Ring-necked Pheasant | 0 |
| Monk Parakeet | 0 |
| *based on susceptibility, mean infectiousness and duration (days) | |
wow such a large table I wish I knew how to collapse it
Infection Responses and Symptoms
Include some current research in each topic, with at least one figure showing data.
Further Reading
[Sample link] Ebola Hemorrhagic Fever—Centers for Disease Control and Prevention, Special Pathogens Branch
References
1. Komar, N., S. Langevin, S. Hinten, N. Nemeth, E. Edwards, D. Hettler, B. Davis, R. Bowen, and M. Bunning. 2003. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerging Infectious Diseases 9(3): 311-322.
2. Greene, S. E., and A. Reid. (2013). FAQ: West Nile Virus, July 2013. American Society For Microbiology.
Edited by Leah Pomerantz, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.