West Nile Virus in Birds: Difference between revisions
| Line 146: | Line 146: | ||
==Effect on Populations== | ==Effect on Populations== | ||
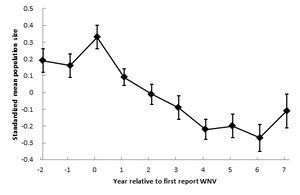
[[Image:leahpomerantzwnv6.jpeg|thumb|300px|left|Crow population changes (± SE) after West Nile virus was first reported in the United States. Data from Koenig et al. [blerg]]]<br> | [[Image:leahpomerantzwnv6.jpeg|thumb|300px|left|Crow population changes (± SE) after West Nile virus was first reported in the United States. Data from Koenig et al. [blerg]]] | ||
West Nile Virus first arose in America in 1999 in New York. | |||
The virus spread across the country - not found in Oregon etc. | |||
Crow populations in particular have been studied and tracked over time. One analysis of populations shows that initially populations declined rapidly, then lessened in decline, and may be back on the rise today. They explain the decrease in decline through the idea of the "dilution effect." They found that areas of higher diversity in bird populations were less affected by the virus. They further hypothesize that as the virus spread across the country, it weakened. etc. etc. | |||
<br> | |||
In late 2012, over two dozen Bald Eagles (<i>Haliaeetus leucocephalus</i> may have succumbed to West Nile Virus infection in Utah. Their deaths caused lots of press coverage because of the irregularity of their deaths, as well as their position as a symbol of the country. The birds were being found laying listless on the ground, many suffering from head tremors, seizures, and paralysis in the legs, feet, and wings. Researchers believe that they may have contracted WNV from eating dead [http://en.wikipedia.org/wiki/Grebe grebes]. Grebes are prevalent in large numbers in the area in the winter months, and may have been affected by an outbreak of WNV. The deaths of these Bald Eagles will most likely not affect the greater population of Bald Eagles in the United States. [xeagles]. | In late 2012, over two dozen Bald Eagles (<i>Haliaeetus leucocephalus</i> may have succumbed to West Nile Virus infection in Utah. Their deaths caused lots of press coverage because of the irregularity of their deaths, as well as their position as a symbol of the country. The birds were being found laying listless on the ground, many suffering from head tremors, seizures, and paralysis in the legs, feet, and wings. Researchers believe that they may have contracted WNV from eating dead [http://en.wikipedia.org/wiki/Grebe grebes]. Grebes are prevalent in large numbers in the area in the winter months, and may have been affected by an outbreak of WNV. The deaths of these Bald Eagles will most likely not affect the greater population of Bald Eagles in the United States. [xeagles]. | ||
Revision as of 06:05, 13 March 2014
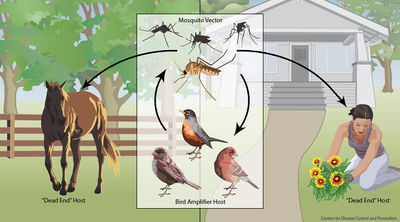
West Nile Virus, of the family Flaviviridae, is a zoonotic disease that infects many species, including humans, throughout the world. Birds are the virus’s primary natural reservoir, where they act as an amplifier host. However, their host competency (their ability to perpetuate the virus' development and spread) and response to infection varies between species; they can have diverging viral loads, viremia, viral shedding, clinical signs, and morbidity. Many factors may affect a particular organism and/or species’ susceptibility and response to infection, including their immune-response capabilities, previous immunities, breeding systems, genetics, geographic spread, and stress levels. Overall, birds vary widely in how they are affected by West Nile Virus.
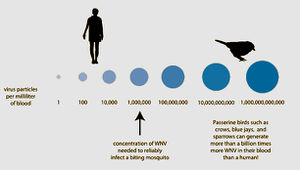
Most mammals, including horses and humans, are “dead-end” hosts for West Nile Virus, as, after infection, the virus does highly concentrate enough in the blood to then be spread to insect vectors. Many bird species, however, are important “amplification-hosts” for the spread of West Nile Virus, as the virus multiplies to exorbitant numbers within them. Some species can generate the highest levels of virus particles in their blood presently known for any viral infection; they can have millions of times more viral particles in their blood than similarly infected mammals. [2 for now]
Initial Infection
Birds may become infected with West Nile Virus in multiple ways. The most common occurrence is through a mosquito vector. However, studies have shown that they may contract the infection through ingestion of infected organisms or direct contact with infected materials.
Insect Vectors
Over 40 species of mosquitoes have been found to carry West Nile Virus. The most important for spread is the genus Culex, which mainly feed on birds. If a mosquito feeds off an infected host with high enough viral load (approximately one million virus particles per milliliter of blood), it will take up the virus into its gut. The virus will then multiply and spread to the rest of the mosquito’s body, including its salivary glands. The vector may then feed off another animal and transmit the virus to a new host.
Other blood sucking insects, such as ticks, have been found to be able to transmit the virus, though they are not as important for spread[2 again].
Oral Transmission
Birds may become infected by West Nile Virus through consuming infected prey items such as insects, small mammals, and other birds. After ingesting infected organisms, viremia usually occurs identically to that of mosquito-borne transmission [xx].
Contact Transmission
Contact Transmission: Research has found that it is possible for birds to transmit the virus to one another as a result of emitting particles in oral or cloacal secretions. These secretions, such as feces, may contaminate water and food, or may directly contact another susceptible organism. In captivity this process is rare, and it is unknown how common it occurs in nature. It is hypothesized that this contact transmission route a factor in spread of the virus in communal roosting populations and during breeding seasons. [1I think, feces.]
Species Susceptibility and Competence
| Table of West Nile Virus host competency of 24 species of birds [1x for now]. | |
| Species | Reservoir Competence Index |
|---|---|
| Blue Jay | 2.55 |
| Common Grackle | 2.04 |
| House Finch | 1.76 |
| American Crow | 1.62 |
| House Sparrow | 1.59 |
| Ring-billed Gull | 1.26 |
| Black-billed Magpie | 1.08 |
| American Robin | 1.08 |
| Red-winged Blackbird | 0.99 |
| American Kestrel | 0.93 |
| Great Horned Owl | 0.88 |
| Killdeer | 0.87 |
| Fish Crow | 0.73 |
| Mallard | 0.48 |
| European Starling | 0.22 |
| Mourning Dove | 0.19 |
| Northern Flicker | 0.06 |
| Canada Goose | 0.03 |
| Rock Dove | 0 |
| American Coot | 0 |
| Japanese Quail | 0 |
| Ring-necked Pheasant | 0 |
| Monk Parakeet | 0 |
Over 300 species of birds have been found infected with West Nile Virus. Passerine birds (of the order Passeriformes, such as flycatchers, robins, wrens, mockingbirds, and finches), charadiiform birds (of the order Charadriiformes, such as gulls, plovers, and stilts), and many raptors have been found to be competent hosts for viral infection and spread. In particular, Corvids (species of the family Corviade, including crows, ravens, magpies, and jays), as well as a few other sensitive species, such as American Kestrels and Great Horned Owls, are competent and highly susceptible. Birds of the orders Anseriformes (ducks, geese), Columbiformes (pigeons), Galliformes (chickens, turkeys), Gruiformes (rails, coots), Piciformes (woodpeckers, toucans), and Psittaciformes (parrots) are generally less competent.
Occurrence of mortality due to infection increases with species competence. In early studies of WNV in the United States, up to 100% mortality was observed in American Crows (Corvus brachyrhynchos) and Blue Jays (Cyanocitta cristata) experimentally inoculated with the virus. In these susceptible species, large amounts of virus are widely distributed in major organs, causing multi-organ failure and inducing a rapid death that does not allow the development of clinical signs [feces]. It is suspected that WNV infection may require underlying illnesses or immune suppression in particular species to result in death. [1x]
It has been found that some species, in particular corvids, such as American Crows, may contain a high enough viremia level that they remain reservoir competent postmortem; they are able to transmit viral particles to vector hosts that feed on their remains up to 5 days after death. [2, xxix]
Many birds that contract West Nile Virus do not die. Certain immune responses, such as antiviral mechanisms, may limit viral infection and viremic spread. Birds with low susceptibility may present minor symptoms and recover, or may appear healthy with low-grade, non-progressive infection. These birds may then have successful immune-mediated clearance of infectious particles. [Xixixi] Some species may also maintain low levels of viral replication that lead to chronic infections [Xiii].
In the wild, birds are frequently found with WNV antibody prevalence. Even American Crows, which experimentally show a very high mortality rate, have been found with WNV antibodies. Thus, individuals vary in their responses to infection.
Infection Symptoms

The majority of individual birds infected with West Nile Virus do not get sick or show symptoms. However, if they do become sick, clinical signs may include uncoordinated walking, lethargy, ataxia, weakness, tremors, inability to fly, anorexia followed by rapid weight loss, green waste products, blindness, lack of awareness, head droop, and abnormal body posture. They usually die within 24 to 48 hours of showing symptoms. In the final phase of infection preceding death, there birds may have severe tremors or seizures. These symptoms reflect virus infection and inflammation of the brain and spinal cord, as well as damage to other tissues, such as the heart, lungs, liver, etc. [xi, xii]
Through bird necropsies, organs found to be most frequently infected with virus particles are the spleen, kidney, skin, and eye. Usually, the virus first appears in the spleen, then rapidly spreads to other organs, and reaches the central nervous system later. Tissue distribution occurs earlier in more susceptible of the bird species. Histologic lesions, such as mild to severe encephalitis, necrosis, inflammation, and hemorrhages, have been found in multiple tissues, including the brain, spinal cord, eye, peripheral nervous system, heart, spleen, liver, kidney, lung, gastrointestinal tract, endocrine system, gonads, skeletal muscle, skin, and bone marrow. The speed of viral distribution and host death may affect the development of lesions, as hosts with higher susceptibility may die before lesions become observable. Macroscopic changes due to infection include emaciation, dehydration, multi-organ hemorrhages, and congestion. [xixix, xiii]
Host Factors
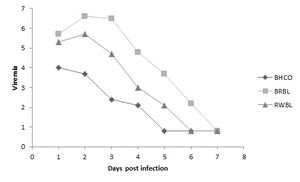
There is little evidence about why bird species are affected by West Nile Viruses differently. Some speculation and study points to life-style differences, such as breeding systems and geographic spread, which lead to unique collections of pathogen defense components for each species. Other differences may arise from genetics, such as intrinsic capabilities to combat viral infection.
Geography
Phylogenetically related, geographically divergent species have been shown to be differently affected by West Nile Virus infection. Thus, their geographic spread is thought to influence their susceptibility. It is believed that this is through climate differences influencing the acquisition of cross-reactive immunologic protection to West Nile Virus. Warmer habitats support greater vector populations as well as better conditions for viral propagation, so southern-living species are more frequently exposed to mosquito born Flaviviridae infections, and thus, develop better resistance to WNV [xiiii].
There is some speculation that differences in species susceptibility may be related to co-evolution of the virus with particular species. In the Old-World (Europe, Africa, Asia), West Nile virus is mostly a benign infection in birds, possibly due to thousands of years of evolutionary selection for birds that produce antibodies and therefore survive. Generally, Old-World birds (such as Barn Owls, Tyto alba, native to Europe) appear to be less susceptible to WNV than New-world birds (such as Red-tailed Hawks, Buteo jamaicensis and Great Horned Owls, Bubo virginianus, native to the Americas). It wasn't until 1999 that the virus first appeared in North America. Therefore, it may be that because Old-World birds have been in contact with the virus for longer, they have evolved better immune responses to it. [xlud, xcorn]
Breeding Systems
Taxonomically similar species may diverge in viral susceptibility due to their type of breeding system. In particular, it has been found that parasitic breeding may increase resistance to WNV, as they present lower viremia levels. Brood parasite bird species, such as Brown-Headed Cowbirds (Molothrus ater), may develop a robust immune system and innate disease response. This is because they are in close contact with varying host bird species, and thus experience infestation with a greater diversity of vector species than do other songbirds. Therefore, this life-style influences their susceptibility to WNV. [xxcow]
Genetics
Few studies look at the genetic basis for species' host capabilities for WNV. However, there has recently been research into a potential genetic basis of WNV resistance in particular bird species. There is a specific gene in chickens, that when expressed in mammalian cells, has antiviral activity against WNV. Thus, this gene may be responsible for chickens’ relative resistance to WNV infection. [xxch]
Effect on Populations
West Nile Virus first arose in America in 1999 in New York. The virus spread across the country - not found in Oregon etc. Crow populations in particular have been studied and tracked over time. One analysis of populations shows that initially populations declined rapidly, then lessened in decline, and may be back on the rise today. They explain the decrease in decline through the idea of the "dilution effect." They found that areas of higher diversity in bird populations were less affected by the virus. They further hypothesize that as the virus spread across the country, it weakened. etc. etc.
In late 2012, over two dozen Bald Eagles (Haliaeetus leucocephalus may have succumbed to West Nile Virus infection in Utah. Their deaths caused lots of press coverage because of the irregularity of their deaths, as well as their position as a symbol of the country. The birds were being found laying listless on the ground, many suffering from head tremors, seizures, and paralysis in the legs, feet, and wings. Researchers believe that they may have contracted WNV from eating dead grebes. Grebes are prevalent in large numbers in the area in the winter months, and may have been affected by an outbreak of WNV. The deaths of these Bald Eagles will most likely not affect the greater population of Bald Eagles in the United States. [xeagles].
Crows decline come back. Allows other species to spread? Fish crows in greater areas.
It could damage endangered species populations.
Future Studies and Surveillance
surveillance systems for dead birds
The USGS (United States Geological Survey) obtains data regarding arboviral cases through an agreement with the Centers for Disease Control and Prevention (CDC) to track cases and develop further knowledge about disease spread. They generally use sentinel chickens for their research [xUSGS].
Further Reading
West Nile Virus—Centers for Disease Control and Prevention
References
1x. Komar, N., S. Langevin, S. Hinten, N. Nemeth, E. Edwards, D. Hettler, B. Davis, R. Bowen, and M. Bunning. 2003. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerging Infectious Diseases 9(3): 311-322.
2. Greene, S. E., and A. Reid. (2013). FAQ: West Nile Virus, July 2013. American Society For Microbiology.
Xii "West Nile Virus." Seattle Audubon Society. Web. 9 Mar. 2014. <http://www.seattleaudubon.org/sas/LearnAboutBirds/SeasonalFacts/WestNileVirus.aspx>.
Xi "Frequently Asked Questions about West Nile Virus and Wildlife." USGS National Wildlife Health Center. N.p., n.d. Web. 9 Mar. 2014. <http://www.nwhc.usgs.gov/disease_information/west_nile_virus/frequently_asked_questions.jsp>.
Xiii Gamino, Virginia, and Ursula Höfle. "Pathology and tissue tropism of natural West Nile virus infection in birds: a review." Veterinary Research 44.1 (2013): 39. Print
Xixixi Nemeth, Nicole, Daniel Gould, Richard Bowen, and Nicholas Komar. "Natural And Experimental West Nile Virus Infection In Five Raptor Species." Journal of Wildlife Diseases 42.1 (2006): 1-13. Print.
xxi. Taylar, Ingrid. Crows. 2012. Seattle, WA. flickr. Web. 9 Mar. 2014. <http://www.flickr.com/photos/taylar/8516674909/>.
xx: Nemeth, Nicole, Daniel Gould, Richard Bowen, and Nicholas Komar. "Natural And Experimental West Nile Virus Infection In Five Raptor Species." Journal of Wildlife Diseases 42.1 (2006): 1-13.
xiiii Lopes, H., P. Redig, A. Glaser, A. Armien, and A. Wünschmann. 2007. Clinical Findings, Lesions, and Viral Antigen Distribution in Great Gray Owls (Strix nebulosa) and Barred Owls (Strix varia) with Spontaneous West Nile Virus Infection. Avian Diseases 51(1): 140-145.
xxix McLean, Robert G., "West Nile Virus: Impact on Crow Populations in the United States" (2004). USDA National Wildlife Research Center - Staff Publications. Paper 367. http://digitalcommons.unl.edu/icwdm_usdanwrc/367
xlud. Ludwig, G.V., P.P. Calle, J.A. Mangiafico, B.L. Raphael, D.K Danner, J.A. Hile, T.L. Clippinger, J.F. Smith, R.A. Cook, T. McNamara. 2002. An outbreak of West Nile virus in a New York City captive wildlife population. American Journal of Tropical Medicine and Hygine 67(1): 67-75.
xcorn. Chu, M., W. Stone, K.J. McGowan, A.A. Dhondt, W.M. Hochachka, and J.E. Therrien. 2003. West Nile File. Birdscope. VOLUME 17, NUMBER 1. Cornell Lab of Ornithology, Web. 11 Mar. 2014. <http://www.birds.cornell.edu/Publications/Birdscope/Winter2003/West_Nile_File.html>.
feces. Kipp A.M., J.A. Lehman, R.A. Bowen, P.E. Fox, M.R. Stephens, K. Klenk, N. Komar, and M.L. Bunning. 2006. West Nile virus quantification in feces of experimentally infected American and fish crows. American Journal of Tropical Medicine and Hygine 75(4): 688-690.
xeagles. Glionna, J.M.. "West Nile virus blamed for rash of bald eagle deaths in Utah." Los Angeles Times 3 Jan. 2014, sec. Nation Now: n/a. Print.
xUSGS. "West Nile Virus Maps - Bird - USA." USGS. U.S. Department of the Interior, n.d. Web. 11 Mar. 2014. <http://diseasemaps.usgs.gov/wnv_us_bird.html>.
Edited by Leah Pomerantz, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.