Wolbachia and The Biological Control of Dengue Virus
By: Elisa Xiao
Introduction
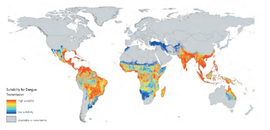
Dengue virus is a mosquito-borne disease that causes an enormous health burden to people living in tropical and subtropical regions. No effective vaccine is yet available. Traditional methods for controlling the spread of mosquito-born disease, such as using bed nets and draining wetlands, failed to control the spread of dengue virus by the Aedes aegytpi mosquitoes because they bit during the days and only occupy urban areas. [1] [2] The transinfection of mosquitos with certain strains of the maternally inherited, endosymbiotic bacteria Wolbachia seems to be a new approach in controlling the spread of dengue virus. Certain strains of this bacterium can invade and sustain themselves in wild mosquito populations, affect mosquito reproduction, reduce lifespan of its host, and interfere with pathogen replication.
Dengue virus
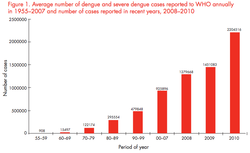
Dengue is the most rapidly spreading mosquito-borne viral disease in the world. According to the latest report by the World Health Organization (WHO), an estimated 50-100 million dengue infections occur annually and approximately 2.5 billion people live in dengue endemic countries. [3] [4]
Structure
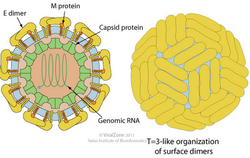
Dengue virus (DENV) is a small single-stranded positive sense RNA virus comprising four distinct serotypes (DENV-1 to -4). The virion of the dengue virus is enveloped, spherical with a diameter of 50nm. The surface proteins are arranged in an icosahedral-like symmetry. The immature virions contain a membrane protein precursor which is cleaved into two virus-encoded membrane proteins (M and E) once it matures. [3] [5]
The vectors
The various serotypes of the dengue virus are transmitted to humans through the bites of dengue-infected Aedes mosquitoes, mainly Aedes aegypti. This mosquito is commonly found in the tropical and subtropical regions and it is widely distributed around the world, mostly between latitudes 35°N and 35°S below an elevation of 1000 metres. These mosquitoes live in water-filled habitats and prefer to live in and around human habitations. [3]
The host
Humans are the primary host of the virus. Although most infections are asymptomatic or subclinical, in severe cases patients can develop plasma leakage, bleeding, or organ impairment in liver, heart and the central nervous system. Primary infection can induce lifelong protective immunity to the infecting serotype, but only short-term immunity to the other serotypes. [3]
Transmission
Female mosquitoes take up dengue virus during feeding on blood of viraemic human. The viruses then infect the female mosquitoes’ mid-gut and replicate in various tissues before infecting the salivary glands, where the virus can then pass on to other humans during feeding. This extrinsic incubation period (EIP) usually takes about 8-12 days.[3]
References
(1) Wilder-SA, Ooi EE, Vasudevan SG, Gubler DJ. 2010. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep 12: 157-164.
(2) Mullard A. 2009. Bacteria could help control dengue fever. Nature News: doi:10.1038
(3) World Health Organization. 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. 2009 edition. France: World Health Organization. ISBN: 978 924 1547871.
(4) World Health Organization. 2012. Global Strategy for dengue prevention and control, 2012-2020. France: World Health Orgainzation. ISBN: 9789241504034.
(5) Zhang W, Chpman PR, Corve J, Johnson PR, Zhang Y, Mukhopadhyay S, Baker TS, Strauss JH, Rossmann MG, Kuhn RJ. 2003. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus.Nat Struct Biol. 11: 907-912.
(6) Rance`s E, Ye YH, Woolfit M, McGraw EA, O’Neill SL. 2012. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLOS Pathog. 8: e1002548.
(7) Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. 2008. How many species are infected with Wolbachia?— A statistical analysis of current data. FEMS Microbiolo Lett 281: 215-220.
(8) Moreira LA, Iturbe-Ormaetex I, Jeffery JA, LU G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limites infection with dengue, Chikungunya, and Plasmodim. Cell 7: 1268-1278.
(9) Turelli M, and Hoffmann AA. 1991. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353: 440-442.
(10) Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O’Neill SL. 2011. Successful establishment of Wolbachia in Aedes popluations to suppress dengue transmission. Nature 476: 454–457.
(11) Min KTai and Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci USA 2: 10792 – 10796.
(12) McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang YF, O’Neill SL. 2009. Stable Introduction of a Life-Shortening Wolbachia Infeciton into the Mosquito Aedes aegypti. Science 2 323: 141-144.
(13) McGraw EA, Merritt DJ, Droller JN, O’Neill SL. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 99:2918-2923.
(14) Salazar M, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. 2007. Dengue virus type 2: replication and tropisms in orally infected Aedesaegypti mosquitoes. BMC Microbiol. 7: 9.
(15) Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL. 2009. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, durin periods of nutritional stress. PLoS Pathog 5: e1000368
(16) Rasgon JL, Styer LM, Scott TW. 2003. Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods. J Med Entomol 40: 125-132.
(17) Bian G, Xu Y, Lu P, Xie Y, Xi Z. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6: e1000833.
(18) Kambris Z, Cook PE, Phuc HK, Sinkins SP. 2009. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326: 134-136.
(19) Heaton NS, Randall G. 2010. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microb 8: 422-432.
(20) Zhang G, Hussain M, O’Neil SL, Asgari S. 2013. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedesaegypti. Proc. Natl. Acad. Sci. USA 110: 10276
(21) Eliminate Dengue Program. 2013. Cairns Field Trial Update – September 2013. Clayton, Victoria, Australia. Retrieved from http://www.eliminatedengue.com/library/publication/document/field_trial_update/20130919_ba_mb_trial_update_handout_combined.pdf
(22) Popovici J, Moreira LA, Poinsignon A, Iturbe-Ormaetxe I, McNaughton D, O’Neill SL. 2010. Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem Inst Oswaldo Cruz 105: 957 – 964
(23) ViralZone:www.expasy.org/viralzone. Swiss Institute of Bioinformatics
(24) Eliminate Dengue Program. School of Biological Sciences, Monash University. http://www.eliminatedengue.com/our-research/dengue-fever
