Yersinia Pestis (Pathogenesis)

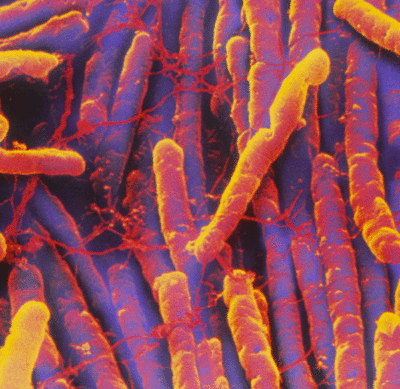
Etiology/Bacteriology
Taxonomy
| Domain = Bacteria | Phylum = Firmicutes | Class = Clostridia | Order = Clostridiales | Family = Clostridiaceae | Genus = Clostridium | species = C. difficile
Description
Pathogenesis
Transmission
Fleas
Y. pestis is most commonly transmitted to humans through the bites of infected fleas, resulting in either primary bubonic plague or septicemic plague (1). In the midgut of its principal flea vector (Xenopsylla cheopis), Y. pestis survives cytotoxic digestion of blood plasma through the action of Yersinia murine toxin (Ymt) (2). Ymt is a plasmid-encoded phospholipase D (PLD) (2). Through the action of this PLD, Y. pestis is able to colonize the flea midgut, which may have caused the bacterium to obligately transmit to arthropods (2). The hemin storage system locus (hms) also contributes to the pathogenicity of Y. pestis in fleas (3). Y. pestis hms encodes for a storage system that synthesizes extracellular saccharides in order to facilitate colonization of the proventriculus in the foregut (3). The formation of biofilms in the proventriculus contributes to the transmission of the plague in fleas (3).
Fluid/Tissue
Y. pestis can be transmitted to humans through the handling of fluids or tissue from infected animals (1). Once Y. pestis has entered the human host, the bacterium spreads throughout the lymphatic system and enters the bloodstream within 2-6 days (4). The spread of Y. pestis throughout the lymphatic system triggers a large-scale immune response with the appearance of buboes on the armpits, groin, and neck (5). Increased numbers of bacteria in the bloodstream promotes the odds of human-human transmission (6). Contact with contaminated fluid or tissue typically results in bubonic plague or septicemic plague (1).
Infectious Droplets
Y. pestis can also be transmitted through the air via infectious droplets from coughing (1). Transmission of infectious droplets is the only method of spreading the plague from person to person (1). Transmission of infectious droplets through coughing enables Y. pestis to colonize the lungs (5). This type of infection is called “pneumonic plague” and has a mortality rate close to 100 percent (1,5).
Infectious dose, incubation, colonization
Epidemiology
Virulence factors
Clinical features
Symptoms
The symptoms of Yersinia Pestis presents in different ways, but the three most common are bubonic, septicemic, and pneumonic plague. [1] Bubonic Plague This type of plague usually results from the bite of an infected flea. Once infection sets, the patient has sudden onset of fever, headache, chills, weakness, and the development of swollen nodes known as buboes, where isolated bacteria multiply and grow. If not treated the bacteria can spread to other areas [1] . Septicemic Plague This type of plague can either develop primarily or as a result from untreated bubonic plague. Symptoms from this include bleeding into the skin and other organs ranging to tissue blackening and death, especially in the fingers, toes, and the nose. [1] Pneumonic Plague This type of plague either develops from inhaling infectious droplets or from untreated bubonic/septicemic plague and bacteria spreading to the lungs. At this point the plague is infectious and can be spread from person to person by infectious droplets. Symptoms from this type of plague include fever, headache, weakness, and a developing pneumonia that heightens symptoms of cough, chest pain and shortness of breath. [1] ===Morbidity and Mortality
Diagnosis
Treatment
Prevention
Host Immune Response
The host innate immune response involves macrophages, inflammation, and the activation of the complement cascade. However, Yersinia Pestis has evolved different mechanisms for evading this immune response, both in the innate and adaptive immune response.
Innate Immune Response
Most of Y. pestis are killed off by encounter with neutrophils and many that survive manage are a special subtype (facultative Y. pestis). Using the macrophages, they are then able to proliferate and express different virulence factors, before the spread systemically throughout the body. In addition, the LPS (lipopolysaccharide) structure in this organism allows the bacteria to become resistant to serum-mediated lysis during its transition from its flea vector to animal host. The bacteria coming from the macrophages, therefore, are resistant to phagocytosis and can inhibit the production of proinflammatory cytokines, which in turn attenuates the adaptive immune response of the host. Yet another immune response that is affected is the complement pathways. The complement cascade of the innate immune response (which is initiated by macrophages binding to foreign antigen) has three different effector functions: opsonization, which leads to phagocytosis, inflammation, and the formation of a membrane-attack complex, which leads to direct killing. However, Y. pestis has developed a resistance to complement-mediated lysis in an effort to survive transmission between flea and animal. In addition, during replication within a macrophage, the bacteria form a needle-like complex that (once released from the macrophage), they use to inject six different effector proteins into different cells to further inhibit the immune response. Targets for this injection include macrophages, dendritic cells, and neutrophils. Besides paralyzing these phagocytic cells, these proteins also target the proinflammatory recruitment response initiated by infected cells. Finally, these proteins also targeting NK cells, which further inhibit the innate immune response.
Adaptive Immune Response
References
1. "Plague Symptoms." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 25 July 2012. Web. 16 July 2014. 1[3]
Created by {Krishna Manohar, Michael Grassi, Christina Cheng, Johnson Ong}, students of Tyrrell Conway at the University of Oklahoma.
