Yersinia pseudotuberculosis infection: Difference between revisions
No edit summary |
|||
| (58 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
{{Conway}} | |||
{{ | |||
[[File:Yersinia_pseudotuberculosis.jpeg|400px|thumb|right| Image of <i>Yersinia psuedotuberculosis</i>. From: tufts.edu [http://bacmap.wishartlab.com/system/images/201/medium/Yersinia_pseudotuberculosis.jpg?1319706244http://sackler.tufts.edu/News/2013/09/New-Insights-into-Yersinia---Host-Interaction]]] | [[File:Yersinia_pseudotuberculosis.jpeg|400px|thumb|right| Image of <i>Yersinia psuedotuberculosis</i>. From: tufts.edu [http://bacmap.wishartlab.com/system/images/201/medium/Yersinia_pseudotuberculosis.jpg?1319706244http://sackler.tufts.edu/News/2013/09/New-Insights-into-Yersinia---Host-Interaction]]] | ||
| Line 10: | Line 8: | ||
| Class = [[Gamma Proteobacteria]] <br> | | Class = [[Gamma Proteobacteria]] <br> | ||
| Order = [[Enterobacteriales]] <br> | | Order = [[Enterobacteriales]] <br> | ||
| Family = | | Family = Enterobacteriaceae <br> | ||
| Genus = [[Yersinia]] <br> | | Genus = [[Yersinia]] <br> | ||
| | | Species = [[Pseudotuberculosis]]<br> | ||
| '''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=109458&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy] Genome: <font size="2">[http://www.ncbi.nlm.nih.gov/genome/?term=yersinia+pseudotuberculosis Genome]</font>''' | | |||
http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi | |||
===Description=== | ===Description=== | ||
<i>Yersinia pseudotuberculosis</i> is a | <i>Yersinia pseudotuberculosis</i> is a Gram-negative, non-lactose fermenting coccobacillus with the capacity to infect both humans and animals, but it is primarily a zoonotic infection [[#References|[1,8]]]. Reported human cases of <i>Y. pseudotuberculosis</i> stem from food and water contamination. This organism possesses high virulence due to the secretion of the superantigenic exotocin YPM. Abdominal pain and fever are ensuing symptoms of this infection, but these are not the sole symptoms. There are a plethora of symptoms that may arise from infection from these bacteria. There is a low fatality rate, despite the large number of complications that may arise from <i>Y. pseudotuberculosis</i>, such as unnecessary appendectomies or bacteremia, which is the presence of organisms into the blood stream [[#References|[3]]]. Diagnosis occurs through analysis of fecal samples, and <i>Y. pseudotuberculosis</i> may be isolated via MacConkey agar. To avoid infection caused by <i>Y. pseudotuberculosis</i>, one should avoid ingesting improperly cooked meat, unpasteurized milk, and contaminated water. In addition, proper hand washing techniques can decrease exposure. | ||
<br> | <br> | ||
==Pathogenesis== | ==Pathogenesis== | ||
===Transmission=== | ===Transmission=== | ||
Although <i> | Although <i>Yersinia pseudotuberculosis </i> is a zoonotic disease, so it can be transmitted to humans. Yersiniosis is primarily caused through infections by <i>Yersinia enterocolitica</i>, but contaminated food and water-borne infections by <i>Y. pseudotuberculosis </i> have been reported as causes. Some examples include water-borne infections are found in Czechoslovakia, as well as, Okayama, Japan. [2] | ||
<br> | |||
Infection in humans begins with the introduction of contaminated food products into the gastrointestinal tract, starting in the intestines and moving into mesenteric lymph nodes. <i>Y. pseudotuberculosis </i> can colonize in a number of different animal reservoirs such as: dogs, cats, cattle, horses, rabbits, deer, turkey, ducks, and many others. | |||
<br> | <br> | ||
===Infectious Dose and Incubation Period=== | ===Infectious Dose and Incubation Period=== | ||
Characteristic of other <i>Yersinia</i> infections, <i>Yersinia pseudotuberculosis</i> requires a dose of | Characteristic of other <i>Yersinia</i> infections, <i>Yersinia pseudotuberculosis</i> requires a dose of 10<sup>9</sup> organisms to cause disease. The incubation period of <i>Y. pseudotuberculosis</i> is 5-10 days; however, durations of 2-20 days have been reported in occasional outbreaks with the average time being 4 days after exposure to the bacterium when symptoms are present. | ||
<br> | <br> | ||
===Epidemiology=== | ===Epidemiology=== | ||
According to the FDA, | According to the FDA, there have been no reported cases of <i>Y. pseudotuberculosis</i> due to food contamination in the United States to date. Sporadic outbreaks of the disease have been reported in Northern Europe and Japan [[#References|[3]]]. In April of 2004, several cases of gastroenteritis due to <i>Y. pseudotuberculosis</i> infection were reported in school children in Finland [[#References|[4]], and contaminated carrots were eventually implicated as the cause of infection. Many other documented cases are due to the consumption of unclean drinking water or contaminated water wells [[#References|[2]]]. Most cases of <i>Y. pseudotuberculosis</i> are isolated and uncommon in humans, as it often causes disease in the animal host. <i>Y. pseudotuberculosis</i> has a low fatality rate in humans, unless the patient presents with chronic liver disease. In this case, the mortality rate can exceed 75% [[#References|[2]]] . Most cases in humans occur in the winter due to the fact that the increased seasonal incidence affects animals. There are also enhanced growth characteristics in cold temperatures [[#References|[13]]] <i>Y. pseudotuberculosis</i> does not appear to have any racial specificities, but it is 3 times more common in men than women. | ||
<br> | <br> | ||
===Virulence Factors=== | ===Virulence Factors=== | ||
<i>Yersinia pseudotuberculosis</i> has a number of virulence factors that contribute to the pathogenicity of the organism. | <i>Yersinia pseudotuberculosis</i> has a number of virulence factors that contribute to the pathogenicity of the organism. The virulence factors of <i>Yersinia pseudotuberculosis</i> are positively correlated with temperature. Specifically, the following virulence factors require an acidic environment at high temperatures to penetrate host cells. As the host's temperature increases, the virulence factors increase in toxicity by allowing them to bind strongly to host lymph tissue. | ||
====Yops==== | ====Yops==== | ||
<i>Y. pseudotuberculosis</i> contains a 70-kd plasmid that encodes for a type III secretion system that delivers the <i>Yersinia</i> outer proteins (Yops). There are four major Yops proteins | <i>Y. pseudotuberculosis</i> contains a 70-kd plasmid that encodes for a type III secretion system that delivers the <i>Yersinia</i> outer proteins (Yops). There are four major Yops proteins that are essential to the pathogenicity of <i>Y. pseudotuberculosis</i>: YopE, YopJ, YopT, and YopH. YopE activates the RhoGTPase of the GTP-binding protein, which plays a role in the actin filament arrangement, promotion of cell rounding, prevention of host cell membrane pores, and inhibition of phagocytosis [[#References|[2]]] . YopE also plays a role in decreasing the host cells pro-inflammatory signals by decreasing the production of interleukin-8. YopJ binds to the protein kinases, which block phosphorylation in the cell. This will eventually lead to a decrease in the production of interleukin-8, affecting the host cells pro-inflammatory response. YopT disrupts the actin filament arrangement and prevents phagocytosis by the host cell. YopT is not present in the pathogenic strains of <i>Y. pseudotuberculosis</i>. YopH also contributes to the disruption of phagocytosis and actin filament arrangement as well as decreases the secretion of interleukin-8. The four main <i>Yersinia</i> outer proteins work together to disrupt the host immune response. | ||
<br> | <br> | ||
====Exotoxin-YPM==== | ====Exotoxin-YPM==== | ||
Some strains of <i>Y. Pseudotuberculosis</i> secrete the superantigenic exotoxin YPM, or <i> Y. pseudotuberculosis</i>-derived mitogen. YPM preferably stimulates the proliferation of CD4 T cells but some expression of CD8 does occur. Along with proliferation, YPM stimulates the overproduction of interleukin-8 increasing the inflammatory response in the host. <br> | Some strains of <i>Y. Pseudotuberculosis</i> secrete the superantigenic exotoxin YPM, or <i> Y. pseudotuberculosis</i>-derived mitogen. YPM preferably stimulates the proliferation of CD4 T cells but some expression of CD8 does occur. Along with proliferation, YPM stimulates the overproduction of interleukin-8, increasing the inflammatory response in the host. <i> Y. pseudotuberculosis </i> strains associated with FESLF were recently genetically sequenced, showing that mobile gene pools contain YPM. YPM includes 3 superantigen--YPMa, YPMb, and YPMc--all of which have pathogenic relevance and differ from other bacterial superantigens [[#References|[13]]]<br> | ||
====Adhesion Molecules==== | ====Adhesion Molecules==== | ||
The adhesion molecules of <i>Y. pseudotuberculosis</i> bind to the host cell and facilitate its colonization in the host organism. The two major proteins of this group include | The adhesion molecules of <i>Y. pseudotuberculosis</i> bind to the host cell and facilitate its colonization in the host organism. The two major proteins of this group include invasin and yadA. The invasin binds to the integrins of the M cells of Peyer’s patch in the small intestine, and it also plays a role in internalization of bacteria across the M cells. YadA binds to laminin, collage, and fibronectin, which are bound to their receptors on the cell surface. <br> | ||
====High Pathogenicity Island (HPI)==== | ====High Pathogenicity Island (HPI)==== | ||
High Pathogenicity Island contains the gene that encodes yersiniabactin, which is used for iron uptake. <br> | High Pathogenicity Island contains the gene that encodes yersiniabactin, which is used for iron uptake. <br> | ||
====Twin Arginine Translocation (tat) pathway==== | ====Twin Arginine Translocation (tat) pathway==== | ||
The twin arginine translocation pathway is important for the secretion of proteins that function in motility and acid resistance. | The twin arginine translocation pathway is important for the secretion of proteins that function in motility and acid resistance. Recent genomic studies have shown that this pathway is similar to the tat pathway in <i> E. coli </i>. It is likely that both of the tat pathways originated from a common promoter. By studying the tat pathway in a much more well known organism such as <i> E. coli </i>, the pathogenicity of the tat virulence factor has become much more well studied. Subsequent findings have proven that the tat protein plays no role in cell growth. Rather, the tat virulence factor remains most coupled to cell motility. <br> | ||
==Clinical Features== | ==Clinical Features== | ||
The two most common symptoms associated with <i>Yersinia pseudotuberculosis</i> infections are abdominal pain and fever. There is no staging process associated with <i>Y. pseudotuberculosis</i> infections | The two most common symptoms associated with <i>Yersinia pseudotuberculosis</i> infections are abdominal pain, which can vary depending on where the bacterium targets the host, and fever. There is no staging process associated with <i>Y. pseudotuberculosis</i> infections. A number of clinical problems can manifest from <i>Y. pseudotuberculosis</i>, including complications in the kidneys, gastrointestinal tract, and severe skin rashes. Associated symptoms may include skin rash, strawberry tongue, and lymphadenopathy. The most common form of <i>Y. pseudotuberculosis</i> infection originates from the ingestion of contaminated foods, especially fresh produce. The ingestion of contaminated food or water, for example, has been shown to cause gastroenteritis, mesenteric lymphadenitis (reference below), and even erythema nodosum. In fact, differential diagnosis can include erythema nodosum and juvenile rheumatoid arthritis [[#References|[13]]] . Diarrhea is uncommon and additional associated symptoms accompany people who develop Izumi fever. | ||
Although infections caused by <i>Y. pseudotuberculosis</i> are broad, the infections are usually self-limited with a low case-fatality rate. | Although infections caused by <i>Y. pseudotuberculosis</i> are broad, the infections are usually self-limited with a low case-fatality rate. [[#References|[5]]] because <i> Y. pseudotuberculosis </i> does not produce iron-binding compounds, patients with iron-overload states such as hemochromatosis,venous congestion, hemolytic anemia, or cirrhosis are at risk for sepsis [[#References|[13]]] . | ||
==Diagnosis== | ==Diagnosis== | ||
<i>Yersinia pseudotuberculosis</i> | <i>Yersinia pseudotuberculosis</i> can be difficult to culture because of the presence of healthy microbiota. A fecal sample is needed from the patient, and then the microorganism can be isolated. Research has shown that cold-temperature enrichment has been effectively used to culture and isolate the microorganism. [[#References|[6]]] Polymerase Chain Reaction assay can then be used to identify the bacteria and then can further serotype the organism. [[#References|[7]]] The culture can also be isolated and grown on MacConkey agar due to its ability to ferment sorbitol and its ability to produce ornithine decarboxylase. [[#References|[8]]] <i>Y. pseudotuberculosis</i> has been serotyped using Enzyme-linked immunosorbent assay along with agglutination tests but the results prove inconclusive due to the possibility of cross-reactions of other pathogenic antibodies. [[#References|[8]]] Blood samples can be taken and tested to confirm the presence of the microorganism but a fecal sample is the preferred method of diagnostic testing. [[#References|[7]]] <br> | ||
==Treatment== | |||
The severity of the infection directly determines the level of treatment that is necessary to clear the colonization of <i>Yersinia pseudotuberculosis</i>. Many cases of <i>Y. pseudotuberculosis</i> are watched closely by a physician, but action is never necessary due to the self-limiting nature of the bacteria. [[#References|[8]]] If necessary, <i>Y. pseudotuberculosis</i> is susceptible to ampicillin, cephalosporins, aminoglycosides, tetracyclines, and chloramphenicol. [[#References|[9]]] If the patient has underlying factors, such as immunodeficiency or severe dehydration, hospitalization may be required so that the correct level of treatment can be administered. [[#References|[8]]] | |||
==Prevention== | |||
Food-borne and water-borne epidemics of <i>Y. pseudotuberculosis</i> can occur. Avoid ingesting uncooked meat, unpasteurized milk, and be aware of the possibilities of contaminated water. Caution should be taken when handling pork intestines especially. Proper hand washing methods should be put in place if ever handling pork in this manner. Be aware when handling animal feces, especially those of livestock. Proper disposal of animal feces is also recommended to avoid water contamination. Again, proper hand washing will prevent the spread of infection from animal to human host. [[#References|[2]]] | |||
==Host Immune Response== | |||
<i>Y. pseudotuberculosis</i> displays unique responses to the host immune system; it is characterized by virulence factors that enable the microorganism to sustain life within the host. One characteristic common to all <i>Yersenia</i> species is the ability to attack phagocytic cells, a crucial part of the host response to pathogenic invasions [[#References|[10]]]. Despite its extracellular residence during pathogenesis, studies have shown that the host’s immune response compensates for the attack on phagocytic cells with CD8+ T cells [[#References|[10]]]. The T cell effector molecule, perforin, plays an equally important role in the death and phagocytosis of the pathogen [[#References|[10]]]. Cell death is characterized by apoptosis of naïve cells and pyroptosis of activated macrophages, which are non-inflammatory and inflammatory, respectively [[#References|[11]]]. <i>Y. pseudotuberculosis</i> is most common in animals, but commonly affects children and immune-compromised individuals when it is transmitted [[#References|[12]]]. | |||
==Damage Response Framework== | |||
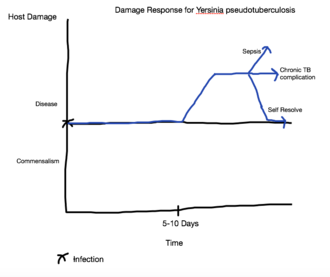
[[Image:KCHWSW2.png|thumb|330px|right|Damage Response Graph for <i> Y. pseudotuberculosis </i>]] | |||
It is common to characterize pathogens based on virulence factors, modes of transmission, or the type of organ systems they infect. One system of classification, called the damage response framework, characterizes microorganisms based on their interactions with the host and the damage that results. [[#References|[14]]] This classification system is based on the principle that the microorganism and the host interact with one another, and that these interactions can be beneficial or harmful. The damage response framework is also based on the idea that the damage that manifests as pathogenic symptoms can be caused by the microorganism or by the host's defenses in response to the microorganism. [[#References|[14]]] Based on this understanding, it is possible to plot the course of the disease, or any other host-microorganism interaction, as a function of host damage versus time. The horizontal line in the middle of the graph represents a neutral interaction between the two factors. An decrease into the lower areas of the graph means the interaction is beneficial to the host, while an increase into the upper regions of the graph indicate damage, even potentially death.[[#References|[14]]] The graph shown here illustrates how the damage response framework can be applied to a <i>Yersinia pseudotuberculosis</i> infection. At the initial moment of infection, generally via a fecal-oral transmission route, there is neither a positive nor a negative affect on the host. [[#References|[8]]] The incubation period <i>Y. pseudotuberculosis </i> can be from 5-10 days.[[#References|[8]]] During this incubation period, little or no damage is done to the host, and, consequently, the damage response does not shift from its neutral position. Following the incubation period <i>Y. pseudotuberculosis </i> can have a 2-20 day latency period, during which time there is little to no damage, so the damage response timeline does not change[[#References|[8]]]. The initiation of symptoms, such as abdominal pain, fever, skin rash, strawberry tongue, and lymphadenopathy, pushes the damage response up quickly, but because the infection is self-limiting, the damage response subsides back to a neutral position between disease and commensalism.[[#References|[8]]] However, the disease can cause chronic complications, in which case the damage response remains elevated due to this long term consequence of the host-microorganism interaction. In more serious cases, <i>Y. pseudotuberculosis </i> can produce sepsis in the host which often leads to death.[[#References|[8]]] This is represented in the damage response timeline by a sharp increase in damage. | |||
==Reference== | |||
1. Long, C., T.F. Jones., D.J. Vugia, J. Scheftel, N. Strockbine, P. Ryan, B. Shiferaw, R.V. Tauxe, L.H. Gould. Yersinia pseudotuberculosis and Y. enterocolitica Infections, FoodNet, 1996–2007. 2010. NCBI. 16(3): 566. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3322025/#__ffn_sectitle> | |||
2. Asim, J. A. "Pseudotuberculosis (Yersinia)". <http://emedicine.medscape.com/article/226871-overview#a0101> | |||
< | |||
3. http://www.fda.gov/Food/FoodborneIllnessContaminants/CausesOfIllnessBadBugBook/ucm070040.htm | |||
4. "<i>Yersinia pseudotuberculosis</i> O:1 Traced to Raw Carrots, Finland." Emerging Infectious Diseases. Vol. 14, No 12, December 2008. <http://wwwnc.cdc.gov/eid/article/14/12/pdfs/08-0284.pdf> | |||
5. Long C, Jones T, Gould L, et al. Yersinia pseudotuberculosis and Y. enterocolitica infections, FoodNet, 1996-2007. Emerging Infectious Diseases [serial online]. March 2010;16(3):566-567. Available from: MEDLINE, Ipswich, MA. | |||
6. Abdulla, Z.A. and Kanan, T.A. "Isolation of Yersinia spp. from cases of diarrhoea in Iraqi infants and children". EMHJ - Eastern Mediterranean Health Journal, 15 (2), 276-284, 2009. <http://apps.who.int/iris/handle/10665/117636#sthash.I4SfU0C4.dpuf> | |||
7. Nakajima, H.; Inoue, M.; Mori, T.; Itoh, K.; Arakawa, E. and Watanabe, H. "Detection and Identification of Yersinia pseudotuberculosis and Pathogenic Yersinia enterocolitica by an Improved Polymerase Chain Reaction Method". Journal of Clinical Microbiology Vol. 30, No.9. September 1992. <http://jcm.asm.org/content/30/9/2484.full.pdf> | |||
8. Jani, A. and Chen, P. "Pseudotuberculosis (Yersinia) Workup". Medscape. November 2013. <http://emedicine.medscape.com/article/226871-workup#a0719> | |||
9. "Yersinia pseudotuberculosis". The Public Health Agency of Canada. 2011. <http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/yersinia-pseudotuberculosis-eng.php#footnote4> | |||
10. Bergman, M.A., W.P. Loomis, J. Mecsas, M.N. Starnbach, and R.R. Isberg. CD8+ T Cells Restrict Yersinia pseudotuberculosis Infection: Bypass of Anti-Phagocytosis by Targeting Antigen-Presenting Cells. 2009. NCBI. 5(9). <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2731216/> | |||
11. Bergsbaken, T., and B.T. Cookson. Innate immune response during Yersinia infection: critical modulation of cell death mechanisms through phagocyte activation. 2009. NCBI. 86(5). <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774879/> | |||
12. Han, T.H., I.K. Pak, and S.J. Kim. Molecular Relatedness between Isolates Yersinia pseudotuberculosis from a Patient and an Isolate from Mountain Spring Water. 2002. NCBI. 18: 425. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3055051/> | |||
13. Asim, A. Jani. Pathophysiology of Y. Pseudotuberculosis. 2013. <http://emedicine.medscape.com/article/226871-overview#a5> <br> | |||
14. Casadevall A, Pirofski L. 2003. The Damage Response Framework of Microbial Pathogenesis. <i>Nature Reviews</i> 10:17-24. | |||
Created by Lindsey Baldwin, Tara Carlisle, Rachel Garrison, and Tory Kappel, students of Dr. Tyrrell Conway at the University of Oklahoma Italian Center | |||
Last edited by Hannah Wilson, Sabrina Waugh, and KC Poe on July 15, 2015 | |||
Latest revision as of 19:15, 11 February 2016

Etiology/Bacteriology
Taxonomy
| Domain = Bacteria
| Phylum = Proteobacteria
| Class = Gamma Proteobacteria
| Order = Enterobacteriales
| Family = Enterobacteriaceae
| Genus = Yersinia
| Species = Pseudotuberculosis
| NCBI: Taxonomy Genome: Genome |
Description
Yersinia pseudotuberculosis is a Gram-negative, non-lactose fermenting coccobacillus with the capacity to infect both humans and animals, but it is primarily a zoonotic infection [1,8]. Reported human cases of Y. pseudotuberculosis stem from food and water contamination. This organism possesses high virulence due to the secretion of the superantigenic exotocin YPM. Abdominal pain and fever are ensuing symptoms of this infection, but these are not the sole symptoms. There are a plethora of symptoms that may arise from infection from these bacteria. There is a low fatality rate, despite the large number of complications that may arise from Y. pseudotuberculosis, such as unnecessary appendectomies or bacteremia, which is the presence of organisms into the blood stream [3]. Diagnosis occurs through analysis of fecal samples, and Y. pseudotuberculosis may be isolated via MacConkey agar. To avoid infection caused by Y. pseudotuberculosis, one should avoid ingesting improperly cooked meat, unpasteurized milk, and contaminated water. In addition, proper hand washing techniques can decrease exposure.
Pathogenesis
Transmission
Although Yersinia pseudotuberculosis is a zoonotic disease, so it can be transmitted to humans. Yersiniosis is primarily caused through infections by Yersinia enterocolitica, but contaminated food and water-borne infections by Y. pseudotuberculosis have been reported as causes. Some examples include water-borne infections are found in Czechoslovakia, as well as, Okayama, Japan. [2]
Infection in humans begins with the introduction of contaminated food products into the gastrointestinal tract, starting in the intestines and moving into mesenteric lymph nodes. Y. pseudotuberculosis can colonize in a number of different animal reservoirs such as: dogs, cats, cattle, horses, rabbits, deer, turkey, ducks, and many others.
Infectious Dose and Incubation Period
Characteristic of other Yersinia infections, Yersinia pseudotuberculosis requires a dose of 109 organisms to cause disease. The incubation period of Y. pseudotuberculosis is 5-10 days; however, durations of 2-20 days have been reported in occasional outbreaks with the average time being 4 days after exposure to the bacterium when symptoms are present.
Epidemiology
According to the FDA, there have been no reported cases of Y. pseudotuberculosis due to food contamination in the United States to date. Sporadic outbreaks of the disease have been reported in Northern Europe and Japan [3]. In April of 2004, several cases of gastroenteritis due to Y. pseudotuberculosis infection were reported in school children in Finland [4, and contaminated carrots were eventually implicated as the cause of infection. Many other documented cases are due to the consumption of unclean drinking water or contaminated water wells [2]. Most cases of Y. pseudotuberculosis are isolated and uncommon in humans, as it often causes disease in the animal host. Y. pseudotuberculosis has a low fatality rate in humans, unless the patient presents with chronic liver disease. In this case, the mortality rate can exceed 75% [2] . Most cases in humans occur in the winter due to the fact that the increased seasonal incidence affects animals. There are also enhanced growth characteristics in cold temperatures [13] Y. pseudotuberculosis does not appear to have any racial specificities, but it is 3 times more common in men than women.
Virulence Factors
Yersinia pseudotuberculosis has a number of virulence factors that contribute to the pathogenicity of the organism. The virulence factors of Yersinia pseudotuberculosis are positively correlated with temperature. Specifically, the following virulence factors require an acidic environment at high temperatures to penetrate host cells. As the host's temperature increases, the virulence factors increase in toxicity by allowing them to bind strongly to host lymph tissue.
Yops
Y. pseudotuberculosis contains a 70-kd plasmid that encodes for a type III secretion system that delivers the Yersinia outer proteins (Yops). There are four major Yops proteins that are essential to the pathogenicity of Y. pseudotuberculosis: YopE, YopJ, YopT, and YopH. YopE activates the RhoGTPase of the GTP-binding protein, which plays a role in the actin filament arrangement, promotion of cell rounding, prevention of host cell membrane pores, and inhibition of phagocytosis [2] . YopE also plays a role in decreasing the host cells pro-inflammatory signals by decreasing the production of interleukin-8. YopJ binds to the protein kinases, which block phosphorylation in the cell. This will eventually lead to a decrease in the production of interleukin-8, affecting the host cells pro-inflammatory response. YopT disrupts the actin filament arrangement and prevents phagocytosis by the host cell. YopT is not present in the pathogenic strains of Y. pseudotuberculosis. YopH also contributes to the disruption of phagocytosis and actin filament arrangement as well as decreases the secretion of interleukin-8. The four main Yersinia outer proteins work together to disrupt the host immune response.
Exotoxin-YPM
Some strains of Y. Pseudotuberculosis secrete the superantigenic exotoxin YPM, or Y. pseudotuberculosis-derived mitogen. YPM preferably stimulates the proliferation of CD4 T cells but some expression of CD8 does occur. Along with proliferation, YPM stimulates the overproduction of interleukin-8, increasing the inflammatory response in the host. Y. pseudotuberculosis strains associated with FESLF were recently genetically sequenced, showing that mobile gene pools contain YPM. YPM includes 3 superantigen--YPMa, YPMb, and YPMc--all of which have pathogenic relevance and differ from other bacterial superantigens [13]
Adhesion Molecules
The adhesion molecules of Y. pseudotuberculosis bind to the host cell and facilitate its colonization in the host organism. The two major proteins of this group include invasin and yadA. The invasin binds to the integrins of the M cells of Peyer’s patch in the small intestine, and it also plays a role in internalization of bacteria across the M cells. YadA binds to laminin, collage, and fibronectin, which are bound to their receptors on the cell surface.
High Pathogenicity Island (HPI)
High Pathogenicity Island contains the gene that encodes yersiniabactin, which is used for iron uptake.
Twin Arginine Translocation (tat) pathway
The twin arginine translocation pathway is important for the secretion of proteins that function in motility and acid resistance. Recent genomic studies have shown that this pathway is similar to the tat pathway in E. coli . It is likely that both of the tat pathways originated from a common promoter. By studying the tat pathway in a much more well known organism such as E. coli , the pathogenicity of the tat virulence factor has become much more well studied. Subsequent findings have proven that the tat protein plays no role in cell growth. Rather, the tat virulence factor remains most coupled to cell motility.
Clinical Features
The two most common symptoms associated with Yersinia pseudotuberculosis infections are abdominal pain, which can vary depending on where the bacterium targets the host, and fever. There is no staging process associated with Y. pseudotuberculosis infections. A number of clinical problems can manifest from Y. pseudotuberculosis, including complications in the kidneys, gastrointestinal tract, and severe skin rashes. Associated symptoms may include skin rash, strawberry tongue, and lymphadenopathy. The most common form of Y. pseudotuberculosis infection originates from the ingestion of contaminated foods, especially fresh produce. The ingestion of contaminated food or water, for example, has been shown to cause gastroenteritis, mesenteric lymphadenitis (reference below), and even erythema nodosum. In fact, differential diagnosis can include erythema nodosum and juvenile rheumatoid arthritis [13] . Diarrhea is uncommon and additional associated symptoms accompany people who develop Izumi fever. Although infections caused by Y. pseudotuberculosis are broad, the infections are usually self-limited with a low case-fatality rate. [5] because Y. pseudotuberculosis does not produce iron-binding compounds, patients with iron-overload states such as hemochromatosis,venous congestion, hemolytic anemia, or cirrhosis are at risk for sepsis [13] .
Diagnosis
Yersinia pseudotuberculosis can be difficult to culture because of the presence of healthy microbiota. A fecal sample is needed from the patient, and then the microorganism can be isolated. Research has shown that cold-temperature enrichment has been effectively used to culture and isolate the microorganism. [6] Polymerase Chain Reaction assay can then be used to identify the bacteria and then can further serotype the organism. [7] The culture can also be isolated and grown on MacConkey agar due to its ability to ferment sorbitol and its ability to produce ornithine decarboxylase. [8] Y. pseudotuberculosis has been serotyped using Enzyme-linked immunosorbent assay along with agglutination tests but the results prove inconclusive due to the possibility of cross-reactions of other pathogenic antibodies. [8] Blood samples can be taken and tested to confirm the presence of the microorganism but a fecal sample is the preferred method of diagnostic testing. [7]
Treatment
The severity of the infection directly determines the level of treatment that is necessary to clear the colonization of Yersinia pseudotuberculosis. Many cases of Y. pseudotuberculosis are watched closely by a physician, but action is never necessary due to the self-limiting nature of the bacteria. [8] If necessary, Y. pseudotuberculosis is susceptible to ampicillin, cephalosporins, aminoglycosides, tetracyclines, and chloramphenicol. [9] If the patient has underlying factors, such as immunodeficiency or severe dehydration, hospitalization may be required so that the correct level of treatment can be administered. [8]
Prevention
Food-borne and water-borne epidemics of Y. pseudotuberculosis can occur. Avoid ingesting uncooked meat, unpasteurized milk, and be aware of the possibilities of contaminated water. Caution should be taken when handling pork intestines especially. Proper hand washing methods should be put in place if ever handling pork in this manner. Be aware when handling animal feces, especially those of livestock. Proper disposal of animal feces is also recommended to avoid water contamination. Again, proper hand washing will prevent the spread of infection from animal to human host. [2]
Host Immune Response
Y. pseudotuberculosis displays unique responses to the host immune system; it is characterized by virulence factors that enable the microorganism to sustain life within the host. One characteristic common to all Yersenia species is the ability to attack phagocytic cells, a crucial part of the host response to pathogenic invasions [10]. Despite its extracellular residence during pathogenesis, studies have shown that the host’s immune response compensates for the attack on phagocytic cells with CD8+ T cells [10]. The T cell effector molecule, perforin, plays an equally important role in the death and phagocytosis of the pathogen [10]. Cell death is characterized by apoptosis of naïve cells and pyroptosis of activated macrophages, which are non-inflammatory and inflammatory, respectively [11]. Y. pseudotuberculosis is most common in animals, but commonly affects children and immune-compromised individuals when it is transmitted [12].
Damage Response Framework
It is common to characterize pathogens based on virulence factors, modes of transmission, or the type of organ systems they infect. One system of classification, called the damage response framework, characterizes microorganisms based on their interactions with the host and the damage that results. [14] This classification system is based on the principle that the microorganism and the host interact with one another, and that these interactions can be beneficial or harmful. The damage response framework is also based on the idea that the damage that manifests as pathogenic symptoms can be caused by the microorganism or by the host's defenses in response to the microorganism. [14] Based on this understanding, it is possible to plot the course of the disease, or any other host-microorganism interaction, as a function of host damage versus time. The horizontal line in the middle of the graph represents a neutral interaction between the two factors. An decrease into the lower areas of the graph means the interaction is beneficial to the host, while an increase into the upper regions of the graph indicate damage, even potentially death.[14] The graph shown here illustrates how the damage response framework can be applied to a Yersinia pseudotuberculosis infection. At the initial moment of infection, generally via a fecal-oral transmission route, there is neither a positive nor a negative affect on the host. [8] The incubation period Y. pseudotuberculosis can be from 5-10 days.[8] During this incubation period, little or no damage is done to the host, and, consequently, the damage response does not shift from its neutral position. Following the incubation period Y. pseudotuberculosis can have a 2-20 day latency period, during which time there is little to no damage, so the damage response timeline does not change[8]. The initiation of symptoms, such as abdominal pain, fever, skin rash, strawberry tongue, and lymphadenopathy, pushes the damage response up quickly, but because the infection is self-limiting, the damage response subsides back to a neutral position between disease and commensalism.[8] However, the disease can cause chronic complications, in which case the damage response remains elevated due to this long term consequence of the host-microorganism interaction. In more serious cases, Y. pseudotuberculosis can produce sepsis in the host which often leads to death.[8] This is represented in the damage response timeline by a sharp increase in damage.
Reference
1. Long, C., T.F. Jones., D.J. Vugia, J. Scheftel, N. Strockbine, P. Ryan, B. Shiferaw, R.V. Tauxe, L.H. Gould. Yersinia pseudotuberculosis and Y. enterocolitica Infections, FoodNet, 1996–2007. 2010. NCBI. 16(3): 566. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3322025/#__ffn_sectitle>
2. Asim, J. A. "Pseudotuberculosis (Yersinia)". <http://emedicine.medscape.com/article/226871-overview#a0101>
3. http://www.fda.gov/Food/FoodborneIllnessContaminants/CausesOfIllnessBadBugBook/ucm070040.htm
4. "Yersinia pseudotuberculosis O:1 Traced to Raw Carrots, Finland." Emerging Infectious Diseases. Vol. 14, No 12, December 2008. <http://wwwnc.cdc.gov/eid/article/14/12/pdfs/08-0284.pdf>
5. Long C, Jones T, Gould L, et al. Yersinia pseudotuberculosis and Y. enterocolitica infections, FoodNet, 1996-2007. Emerging Infectious Diseases [serial online]. March 2010;16(3):566-567. Available from: MEDLINE, Ipswich, MA.
6. Abdulla, Z.A. and Kanan, T.A. "Isolation of Yersinia spp. from cases of diarrhoea in Iraqi infants and children". EMHJ - Eastern Mediterranean Health Journal, 15 (2), 276-284, 2009. <http://apps.who.int/iris/handle/10665/117636#sthash.I4SfU0C4.dpuf>
7. Nakajima, H.; Inoue, M.; Mori, T.; Itoh, K.; Arakawa, E. and Watanabe, H. "Detection and Identification of Yersinia pseudotuberculosis and Pathogenic Yersinia enterocolitica by an Improved Polymerase Chain Reaction Method". Journal of Clinical Microbiology Vol. 30, No.9. September 1992. <http://jcm.asm.org/content/30/9/2484.full.pdf>
8. Jani, A. and Chen, P. "Pseudotuberculosis (Yersinia) Workup". Medscape. November 2013. <http://emedicine.medscape.com/article/226871-workup#a0719>
9. "Yersinia pseudotuberculosis". The Public Health Agency of Canada. 2011. <http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/yersinia-pseudotuberculosis-eng.php#footnote4>
10. Bergman, M.A., W.P. Loomis, J. Mecsas, M.N. Starnbach, and R.R. Isberg. CD8+ T Cells Restrict Yersinia pseudotuberculosis Infection: Bypass of Anti-Phagocytosis by Targeting Antigen-Presenting Cells. 2009. NCBI. 5(9). <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2731216/>
11. Bergsbaken, T., and B.T. Cookson. Innate immune response during Yersinia infection: critical modulation of cell death mechanisms through phagocyte activation. 2009. NCBI. 86(5). <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774879/>
12. Han, T.H., I.K. Pak, and S.J. Kim. Molecular Relatedness between Isolates Yersinia pseudotuberculosis from a Patient and an Isolate from Mountain Spring Water. 2002. NCBI. 18: 425. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3055051/>
13. Asim, A. Jani. Pathophysiology of Y. Pseudotuberculosis. 2013. <http://emedicine.medscape.com/article/226871-overview#a5>
14. Casadevall A, Pirofski L. 2003. The Damage Response Framework of Microbial Pathogenesis. Nature Reviews 10:17-24.
Created by Lindsey Baldwin, Tara Carlisle, Rachel Garrison, and Tory Kappel, students of Dr. Tyrrell Conway at the University of Oklahoma Italian Center
Last edited by Hannah Wilson, Sabrina Waugh, and KC Poe on July 15, 2015